Abstract
Aims: Defining the clinical and physiologic significance of an intermediate coronary artery stenosis is aided by measurement of fractional flow reserve (FFR). Adenosine is the most common agent used in the cardiac catheterisation laboratory for the measurement of FFR. Regadenoson, a selective adenosine receptor agonist, with fewer side effects than adenosine has been used extensively in stress testing to induce hyperaemia. We postulated that FFR measurements would be equivalent following administration of regadenoson and adenosine.
Methods and results: Twenty patients with an angiographic intermediate coronary artery stenosis (50% to 80%) were included in the study. FFR was measured during three minutes of intravenous (IV) adenosine infusion and for five minutes after an injection of regadenoson. The mean difference between the FFR measured by IV adenosine and IV regadenoson was 0.0040 (min -0.04, max +0.04, standard deviation [SD] 0.025). There was a strong linear correlation between the FFR measured by IV adenosine and IV regadenoson (R2 linear=0.933). The FFR at maximum hyperaemia was achieved earlier using regadenoson than adenosine (59±24.5 sec vs. 93±44.5 sec, p=0.01).
Conclusions: Regadenoson produces similar pressure-derived FFR compared to IV adenosine infusion.
Introduction
Coronary artery angiography is frequently limited in its ability to define the clinical or physiological significance of coronary artery stenoses of intermediate severity. Fractional flow reserve (FFR) is useful in defining the physiological significance of intermediate stenoses. Percutaneous coronary interventions (PCI) guided by FFR and angiography have better outcomes than PCI guided by angiography alone in single-vessel and multivessel disease1,2. Fractional flow reserve is defined as the ratio of maximum flow in the presence of a stenosis to the theoretical normal maximum flow in the same coronary artery3. It is easily measured in the cardiac catheterisation laboratory as the ratio of the mean coronary pressure distal to the stenosis (Pd) to the mean coronary pressure proximal to the stenosis (Pa) in conditions of maximal hyperaemia (FFR=Pd/Pa). An FFR value <0.80 is associated with a haemodynamically significant stenosis2,4.
Coronary hyperaemia is most commonly induced by intracoronary or IV adenosine4. Intracoronary (IC) papaverine is an excellent agent for achieving maximum hyperaemia but there is concern that the associated QT interval prolongation might lead to ventricular tachycardia4,5. Adenosine is a non-selective agonist of the A1, A2a, A2b and A3 receptors. Activation of adenosine A2a receptors in the vascular endothelium causes coronary hyperaemia. Adenosine A1, A2b and A3 receptor activation results in associated side effects, including negative inotropic and chronotropic responses, mast cell degranulation and bronchospasm with frequent symptoms of chest pain, shortness of breath, facial flushing and headache6.
Regadenoson is the first selective adenosine A2a receptor agonist approved by the US Food and Drug Administration (FDA)7. Regadenoson is now used extensively in pharmacologic myocardial perfusion imaging7.
The aim of the current study was to compare the effects of fixed dose regadenoson and IV adenosine on FFR measurements. We tested the hypothesis that fixed dose regadenoson and IV adenosine would produce similar FFR responses.
Methods
STUDY POPULATION
From October 2009 to September 2010, 67 patients referred to the Albert Einstein Medical Center Cardiac Catheterization Laboratory for coronary angiography gave initial consent prior to their coronary angiogram. Once the coronary angiography was performed, patients with intermediate coronary artery disease requiring FFR evaluation were identified and administered adenosine and regadenoson as per the pharmacologic protocol. Twenty patients had an intermediate coronary artery lesion, defined as 50 to 80% diameter stenosis of a major coronary artery. Patients were excluded if they had had an ST-segment elevation myocardial infarction within the previous five days, significant left main coronary artery stenosis, heart block, pregnancy, asthma or hypersensitivity to either adenosine and/or regadenoson. All patients provided informed consent. The protocol complies with the Declaration of Helsinki and was approved by the Institutional Review Board at Albert Einstein Medical Center. The study met the exemption requirements under 21CFR 312.2 of the investigational new drug (IND) application, and IND exemption was received for the protocol from the FDA prior to starting the study8.
CATHETERISATION PROTOCOL
After diagnostic coronary cineangiography and identification of an intermediate coronary artery lesion, a guiding catheter was manoeuvred to the coronary ostium. Attention was paid to avoid arterial pressure wave damping. Intracoronary nitroglycerine was injected at a dose of 100-200 mcg into the coronary artery with the intermediate coronary artery lesion. A 0.014 inch-diameter, high-fidelity pressure-recording guidewire (PressureWireTM; Radi Medical Systems, Uppsala, Sweden) was externally calibrated and then advanced to the distal tip of the catheter. With the catheter outside the ostium of the vessel, equalisation of pressures of the catheter and the pressure wire was verified. The pressure wire was advanced into the coronary artery with the pressure sensor placed beyond the lesion. The guiding catheter was withdrawn from the coronary ostium. Beat to beat measurements of distal coronary artery and simultaneous aortic pressures were made at baseline and at maximal hyperaemia. At the end of the study, the pressure wire was brought back near the distal tip of the guiding catheter and equalisation of catheter and pressure wire was verified.
PHARMACOLOGIC PROTOCOL
Intravenous access was obtained prior to cardiac catheterisation with an 18 gauge needle through the right or left antecubital vein. All patients received an initial IV infusion of adenosine first at a dose of 175 μg/kg through a peripheral IV line for three minutes. The IV access site was flushed with 10 cc of normal saline to remove any residual adenosine. After a washout period of five minutes, a bolus of regadenoson 0.4 mg was given through the same peripheral IV line. The proximal aortic pressure, distal coronary pressure, FFR, heart rate, systolic blood pressure and diastolic blood pressure were recorded during 0, 1, 2 and 3 minutes of adenosine infusion and 0, 1, 2, 3, 4 and 5 minutes after regadenoson injection. The lowest value of FFR anytime during the three minutes of adenosine infusion and during the five minutes after regadenoson injection, and the time at which this was achieved, were recorded. During the washout period after adenosine infusion, heart rate, systolic blood pressure and diastolic blood pressure were recorded for five minutes. The value of FFR measured during adenosine infusion was used to guide PCI appropriateness.
STATISTICAL ANALYSIS
Linear regression was used to show the relationship between the FFR with adenosine versus regadenoson. The paired t-test was used to compare the mean times to achieve FFR, mean arterial pressure (MAP), change in MAP, heart rate, and change in heart rate. All means were reported as mean±standard deviation (SD). Statistical significance was defined as p<0.05. The Bland-Altman plot was used to show agreement between the FFR using adenosine and the FFR using regadenoson. A range of agreement was defined as mean±SD. All analysis was performed using SPSS 14.0.0 (SPSS, Inc., Chicago, IL, USA) software.
Results
From October 2009 to September 2010, a total of 67 patients were recruited for this study of which 20 met criteria for the study with an intermediate coronary artery stenosis estimated as a 50-80% stenosis amenable to FFR measurement. Mean age was 63±9 years. There were 16 males and four females. The procedural success was 100%. During the five minutes after the termination of adenosine infusion all patients’ FFR values returned to baseline prior to injection of regadenoson. In contrast only three out of 20 patients returned to baseline five minutes after regadenoson IV injection. The change in mean arterial blood pressure and heart rate at various time points after IV adenosine and IV regadenoson are shown in Table 1.
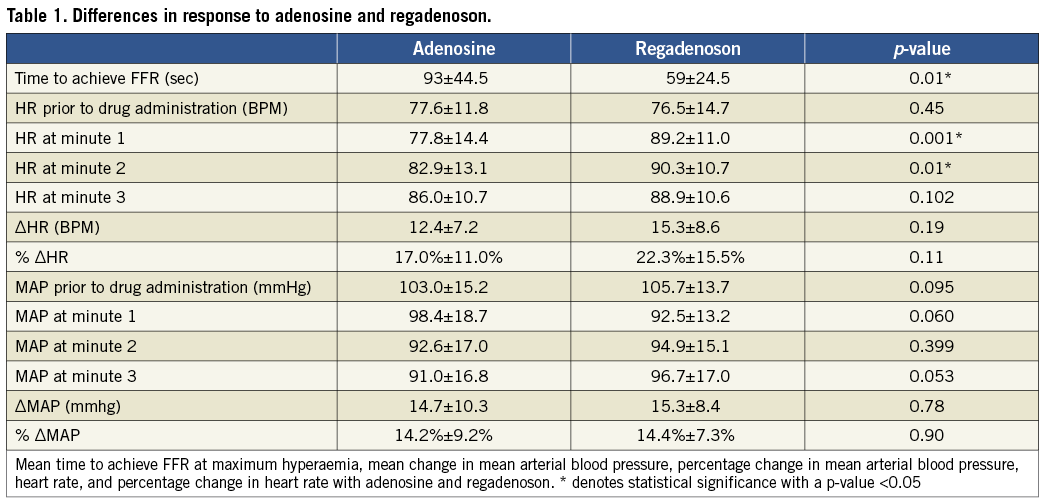
INTRAVENOUS ADENOSINE VERSUS REGADENOSON FOR CALCULATING PRESSURE- DERIVED FFR MEASUREMENTS
The mean FFR measured by IV adenosine and IV regadenoson was 0.84±0.08 and 0.84±0.09, respectively. The mean difference between the FFR measured by IV adenosine and IV regadenoson was 0.0040 (min –0.04, max +0.04, SD 0.025). There were no FFR measurements differing >0.04 between IV adenosine and IV regadenoson. There was a strong linear correlation between the FFR measured by IV adenosine and IV regadenoson (y=1.0963x–00.0849, R2 linear=0.933, p<0.001) (Figure 1). The agreement between the two sets of measurements was excellent (Figure 2).
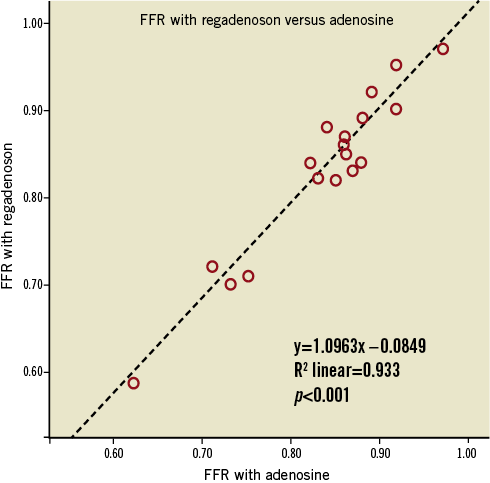
Figure 1. Linear regression analysis of FFR measurements performed with IV regadenoson (dependent variable) and IV adenosine (independent variable). There are only 18 data points as two pairs of patients had similar values.
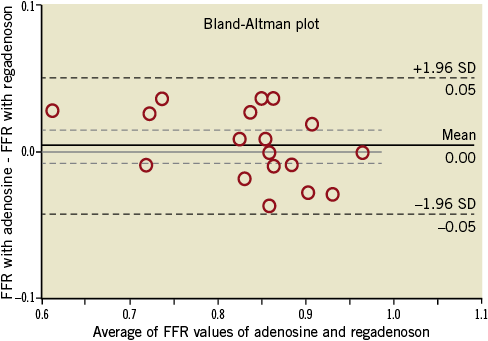
Figure 2. Bland-Altman agreement between two sets of measurements. Difference between measurements with IV regadenoson and IV adenosine plotted against mean. There are only 18 data points as two pairs of patients had similar values.
TIME TO ACHIEVE FFR (Table 1)
The regadenoson injection induced maximal hyperaemia faster than adenosine. The mean time to achieve FFR at maximum hyperaemia with adenosine was 93±44.5 seconds and with regadenoson 59±24.5 seconds (p=0.01).
EFFECT ON BLOOD PRESSURE AND HEART RATE (Table 1)
Administration of both adenosine and regadenoson increased the heart rate (12.4±7.2 vs. 15.3±8.6 beats per minute [BPM], p=0.19). There was no significant difference in the percent change in heart rate between adenosine and regadenoson (17.0%±11.0% vs. 22.3%±15.5%, p=0.11). The heart rate increased earlier following regadenoson than adenosine (Figure 3).
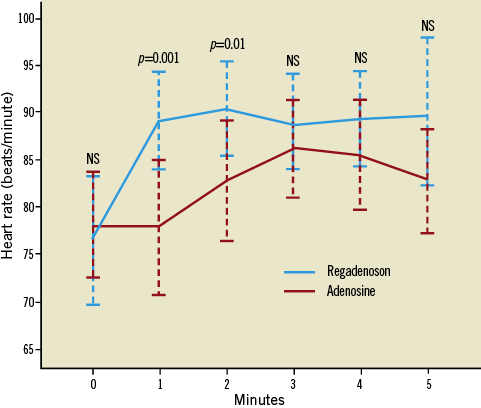
Figure 3. Change in heart rate with adenosine and regadenoson. Graph represents mean heart rate with 95% confidence interval.
Administration of both adenosine and regadenoson decreased mean arterial pressure (MAP) after administration (14.7±10.3 mmHg vs. 15.3±8.4 mmHg, p=0.78). There was no significant difference in the percent change in MAP between adenosine and regadenoson (14.2%±9.2% vs. 14.4%±7.3%, p=0.90) (Figure 4).
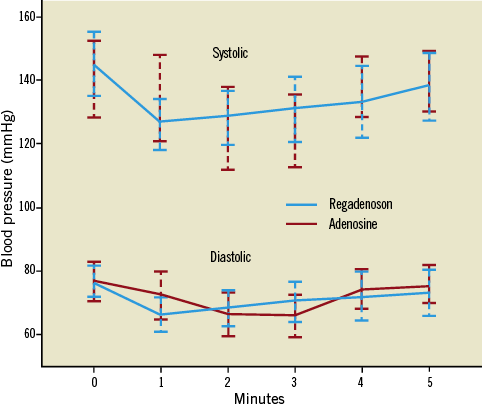
Figure 4. Change in central aortic systolic and diastolic blood pressure with adenosine and regadenoson. Graph represents mean heart rate with 95% confidence interval.
ADVERSE REACTIONS
Two patients developed transient complete heart block while receiving IV adenosine; however, these episodes did not require intervention. There were no cases of complete heart block with regadenoson. There was no bronchospasm or severe chest pain with adenosine or regadenoson administration.
Discussion
The findings of this study show that an IV injection of regadenoson results in an equivalent pressure-derived FFR compared to an IV infusion of adenosine. The FFR at maximum hyperaemia was achieved earlier with regadenoson. Both adenosine and regadenoson were associated with similar effects on heart rate and absolute decrease in MAP. Two patients (10%) developed heart block with adenosine while none of the patients experienced heart block with regadenoson.
PCI guided by FFR is associated with better outcomes than PCI guided by angiography alone1,2. FFR measures the fraction of maximal coronary flow reduced by the presence of a stenosis. Maximal flow in the coronary artery can be achieved by exercise and pharmacological means. The pharmacological methods of hyperaemia include IV papaverine, adenosine, and adenosine-5’-triphosphate4. Papaverine has been reported to cause ventricular tachycardia associated with prolongation of the QT interval. Papaverine is still used in some centres as the incidence of ventricular tachycardia is rare. Adenosine-5’-triphosphate is used in Japan but is not approved for use in the United States. In the United States the primary method of induction of hyperaemia is adenosine whether by IV or IC administration. Adenosine activates adenosine A1, A2a, A2b and A3 receptors. Adenosine, through its action on adenosine A1, A2b and A3 receptors, causes side effects including shortness of breath, facial flushing, chest pain, headache, bradyarrhythmias and heart blocks.
Regadenoson is the first selective A2a receptor agonist that has been approved by the FDA and is currently used clinically for pharmacological myocardial perfusion imaging. In a study of 38 volunteers after boluses of regadenoson there was a threefold increase in coronary flow velocity9. Regadenoson has several potential advantages over IV adenosine for the measurement of FFR. Regadenoson has a rapid onset of action, has a similar but reduced side effect profile compared to adenosine, and side effects if severe can be reversed with aminophylline10. Regadenoson is administered as a single IV bolus through a peripheral line of 400 µg over 10 seconds. Since regadenoson dosing is not weight-based this reduces the chance of error and inadequate dosing10. Patients who weigh more than 72 kg usually require more than one vial of adenosine (Adenoscan®; Astellas Pharma US, Inc., Deerfield, IL, USA) thereby doubling the cost of adenosine and making regadenoson more cost-effective. Regadenoson is administered as an IV bolus injection and therefore does not need preparation of an IV line and IV infusion pump. This will potentially reduce the total procedure time, procedure costs and also patient discomfort. Regadenoson has a longer duration of action but the maximum hyperaemic response is brief and thus may not be useful for FFR evaluation of serial stenosis by pullback analysis and multivessel disease.
Recently there has been one other study published comparing adenosine with regadenoson for the measurement of FFR11. This study even though similar to our study has some major differences. The prior study followed the ADVANCE-3 trial inclusion and exclusion criteria while our study had very few exclusion criteria, and therefore included a more typical population of patients encountered in clinical practice10. The use of a 140 mcg/kg/min adenosine dose through a peripheral IV line in the prior study has been associated with peripheral inactivation of adenosine. In our study we used a higher dose of adenosine –175 mcg/kg/min– to mitigate this effect.
The publication of the ADenosine Vasodilator Independent Stenosis Evaluation (ADVISE) study may make the instantaneous wave-free ratio (iFR) a viable and interesting alternative for a drug-free option to evaluate haemodynamic stenosis. For now, pharmacological methods for FFR are still the standard of care and during this time regadenoson may play a role12.
Limitations of this study
This is a single-centre study of 20 patients. Moreover, this is not a blinded study. Currently the gold standard for FFR measurement is continuous IV infusion of adenosine through a central vein, but IV infusion of adenosine through a peripheral line is the most common method of FFR measurement performed in the United States, and therefore to reflect common clinical practice this method was chosen. We used a higher dose of adenosine to overcome this concern. All patients received adenosine first and then regadenoson. It is possible that residual hyperaemia from adenosine at the time of regadenoson injection was present but this is unlikely as there was a five-minute washout period prior to regadenoson injection and all haemodynamic characteristics returned to baseline before regadenoson injection. Patients were not given regadenoson first as the hyperaemia due to regadenoson persists for much longer and it would have been technically difficult to wait long enough for the hyperaemia from regadenoson to resolve. Adenosine was infused for three minutes; the incidence of heart block with adenosine infusion might potentially have been reduced if it had been stopped at maximal hyperaemia and not continued for the entire three minutes. It is common clinical practice in many centres measuring FFR to infuse adenosine for at least three minutes and we designed the study to reflect this.
Conclusions
Regadenoson produces similar pressure-derived FFR compared to IV adenosine infusion. Obvious advantages include single bolus, non-weight-dependent dosing and decreased side effects. To our knowledge there has been only one other published study comparing regadenoson with adenosine for FFR measurements11.
Funding sources
This work was funded in part by the Women’s League for Medical Research, York & Tabor Rds, Philadelphia, PA 19102, USA.
Conflict of interest statement
The authors have no conflicts of interest to declare.

