Coronary artery physiology assessment using hyperaemic or non-hyperaemic indices has been extensively used to guide percutaneous coronary intervention (PCI) while post-stenting appears to provide useful prognostic information and predict future adverse cardiovascular events. Several studies have attempted, in recent years, to identify predictors of post-PCI physiology and the effect of stenting on patients’ quality of life. The most promising is the hyperaemic pullback pressure gradient (PPG) index, which takes into account the maximum pressure gradient in a 20 mm segment, the total pressure gradient across the lesion and lesion length to classify coronary artery disease (CAD) as focal or diffuse1. Reports have shown that the PPG index, estimated using either fractional flow reserve (FFR) or angiography-derived FFR, can predict post-PCI FFR, target lesion failure2, and the effect of the procedure on patients’ symptoms and quality of life3. More interestingly, a post hoc analysis of the P3 Study (ClinicalTrials.gov: NCT03782688) demonstrated that CAD physiology patterns were associated with plaque phenotypes, with the lesions with focal disease having increased plaque burden (PB) and vulnerable lipid-rich morphology, while those with diffuse disease exhibited a more stable phenotype and increased calcific burden4.
The study published by Kotoku et al in this issue of EuroIntervention, provides additional insights about the association between plaque morphology and pathophysiology derived by the PPG index5. The authors analysed data from 206 patients with a chronic coronary syndrome who were recruited in the ASET-JAPAN study. They assessed coronary physiology patterns, using Murray law-based quantitative flow ratio (μQFR) indices, and plaque composition in stented and non-stented segments, using intravascular ultrasound (IVUS) or optical coherence tomography (OCT) imaging, pre- and post-PCI5. In line with previous reports, they demonstrated that diffuse CAD is a predictor of an unfavourable physiological outcome post-PCI (μQFR <0.91), which was attributed to a pressure drop proximal and distal to the treated segment, while the intrastent pressure gradient was not different between groups at the end of the procedure. Intravascular imaging of the treated segments pre-PCI showed an increased PB in the diffuse disease group, while post-PCI, the reference lumen and minimum stent areas were smaller in this group; there was no difference in the stent expansion between groups. As expected, patients with diffuse disease had a larger PB and smaller lumen area in the non-stented segments compared to patients in the focal disease group, while IVUS greyscale pixel intensity analysis did not show differences in plaque characteristics in the two groups.
The manuscript is of great interest, as it provides additional insights about the implications of the baseline pathophysiological patterns on the post-PCI FFR and the interplay between plaque physiology and morphology. The large number of patients included, the serial intravascular imaging performed in many of the patients, and the thorough IVUS and OCT analyses are advantages of the study. However, it also has significant limitations that should be acknowledged. Firstly, the authors used μQFR rather than invasive hyperaemic or non-hyperaemic indices to assess coronary physiology. Secondly, most of the patients had IVUS-guided revascularisation; this modality enables accurate quantification of the PB, but it has limitations in assessing plaque composition compared to OCT or near-infrared spectroscopy-IVUS (NIRS-IVUS)6. Thirdly, intravascular imaging and echogenicity analysis were performed pre-PCI in 64% and 44% of the treated vessels, respectively, while post-PCI, the proximal non-stented segment was analysed in 52% of these segments and the distal in 68%, while echogenicity was available in 32% and 45% of these segments, respectively. Finally, intravascular imaging assessed only 11.5 mm of the proximal segment and 9.1 mm of the distal segment, and not the entire vessel.
The findings of this analysis challenge the post hoc analysis of the P3 study linking physiology patterns with plaque phenotypes and, in particular, focal disease with a vulnerable plaque morphology4. The limitations of the present study and of the previous report (i.e., the small number of patients included, the use of OCT in 57% of the patients, and more importantly, the significant differences in the severity of CAD with a smaller minimum lumen area, lower FFR values and a higher PB in the focal disease group, which are all well-established predictors of plaque vulnerability) (Figure 1)7 do not allow us to draw safe conclusions about the association between plaque pathology and physiology4.
It has been argued that PPG may regulate plaque progression, as an increased pressure gradient across a lesion has been considered a trigger of plaque destabilisation8. However, the value of PPG to capture vessel microphysiology and the distribution of the local haemodynamic forces without taking into account plaque geometry, its longitudinal and circumferential distribution or the side branches that critically determine shear and plaque structural stress patterns has not been tested yet. In addition, the role of this metric to predict plaque morphology and more importantly, its natural evolution, requires further evaluation910. In contrast to both studies that included severely stenotic lesions, research should focus on non-flow-limiting lesions that are exposed to smaller pressure gradients and left untreated. The existing evidence in this setting from all the prospective studies of coronary atherosclerosis suggests that lesion length is not a predictor of events; however, the PPG hypothesis is an interesting concept and needs to be tested.
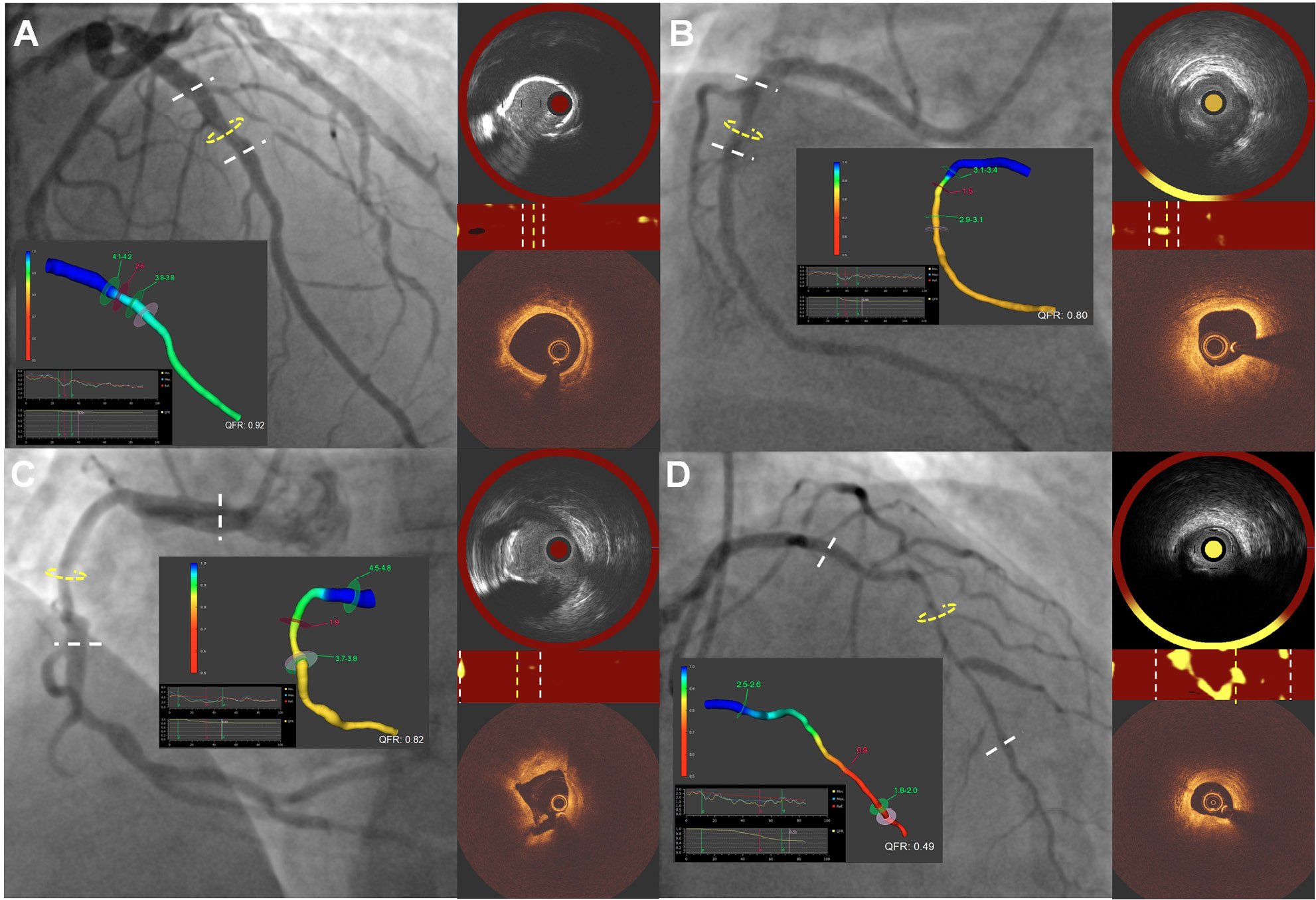
Figure 1. Case examples of moderate and advanced CAD with a diffuse and focal pathophysiological pattern underscoring the importance of lesion severity on plaque composition. A) A focal non-flow-limiting lesion (QFR: 0.92, PPG index derived by QFR: 0.83) in the mid-LAD with NIRS-IVUS and OCT imaging at the MLA – indicated with a yellow circle in the angiogram and a yellow line in the chemogram; the proximal and distal reference segments of the lesion are shown with white lines – demonstrating a calcified plaque and no lipid; B) a focal flow-limiting stenosis (PPG index: 0.91, QFR: 0.80) in the mid-RCA, with a small MLA, a large PB and a fibroatheroma phenotype; C) a diffuse non-flow-limiting stenosis (PPG index: 0.68, QFR: 0.82) in the mid-RCA, with a large MLA, increased calcific burden and no lipid; and D) a critical diffuse stenosis (QFR: 0.49; PPG index: 0.31) involving the entire LAD, with a large PB and lipid content. CAD: coronary artery disease; IVUS: intravascular ultrasound; LAD: left anterior descending artery; MLA: minimum lumen area; NIRS: near-infrared spectroscopy; OCT: optical coherence tomography; PB: plaque burden; PPG: pullback pressure gradient; QFR: quantitative flow ratio; RCA: right coronary artery
Conflict of interest statement
The authors have no conflicts of interest to declare.

