Abstract
Background: The best criteria for adequate stent expansion assessment by intracoronary imaging remain debated and their correlation with post-PCI FFR values is unknown.
Aims: This study aimed to analyse the relationship between stent expansion criteria using optical coherence tomography (OCT) analysis and the final PCI functional result.
Methods: This post hoc analysis of the DOCTORS study included non-ST-elevation segment ACS patients undergoing OCT-guided PCI. The procedure functional result was assessed by the measurement of fractional flow reserve (FFR). Stent expansion was assessed on OCT runs according to the DOCTORS criteria and ILUMIEN III criteria.
Results: The study included N=116 patients (age: 60.8±11.5 years; male gender: 71%). The final expansion was considered optimal in 10%, acceptable in 9% and unacceptable in 81% of the stents according to ILUMIEN III criteria, although being successful in 70% of the patients according to the DOCTORS criteria. Hypertension and larger proximal reference segment dimension were independent predictors of inadequate device ILUMIEN III expansion. FFR values were, respectively, 0.93 (0.91-0.95) versus 0.95 (0.92-0.97) in patients with optimal+acceptable versus unacceptable ILUMIEN III expansion (p=0.22), 0.94 (0.91-0.97) versus 0.95 (0.93-0.97) in patients with optimal versus non-optimal DOCTORS expansion (p=0.23), and 0.95 (0.92-0.97) versus 0.92 (0.90-0.95) in patients with minimal stent area ≥4.5 mm2 versus <4.5 mm2 (p=0.03).
Conclusions: In this selected population, no relationship was observed between optimal stent expansion according to ILUMIEN III or DOCTORS OCT criteria and final post-PCI FFR values.
Introduction
Although angiographic guidance is the established standard of care during percutaneous coronary intervention (PCI), optical coherence tomography (OCT) offers potential advantages compared with angiography for evaluation of lesion characteristics and procedural outcome optimisation, including stent expansion1,2. Stent underexpansion has been identified as a major mechanical trigger for acute/subacute device thrombosis and a determinant for future restenosis2,3,4. Though several expansion criteria have been proposed by intravascular ultrasound (IVUS) studies, they are still debated and OCT data investigating this field are scarce2. The ILUMIEN III trial recently compared OCT, IVUS and angiography-guided PCI strategies for coronary artery lesion revascularisation. The authors proposed and applied a specific protocol to establish stent length, diameter, and expansion according to the reference segment, using a two-section analysis to define optimal deployment5. Nevertheless, there is no evidence that PCI optimisation according to this methodology could improve clinical outcomes; attempts to achieve optimal expansion could even favour complications. Furthermore, whether stent expansion assessed by OCT (optimal or not) could influence post-procedural fractional flow reserve (FFR) values remains to be determined.
The DOCTORS (Does Optical Coherence Tomography Optimize Results of Stenting) multicentric study randomised n=240 patients to angiography-guided PCI or OCT-guided PCI with the post-PCI FFR value as the primary endpoint6,7. In this latter group we investigated the impact of adequate stent expansion assessed by OCT and different criteria on the physiological result of the PCI.
Material and methods
STUDY DESIGN
This study is a post hoc analysis of data from the DOCTORS multicentre randomised prospective study. The DOCTORS study evaluated whether the use of OCT during PCI would provide useful clinical information and modify physician decision making, thus affecting the functional result of angioplasty as assessed by FFR measured after stent implantation in a lesion responsible for non-ST-elevation acute coronary syndrome (NSTE-ACS). The design and the main results of the trial have been detailed elsewhere6,7. The study protocol was approved by the Institutional Review Board of the University Hospital of Besançon, France, and all participants provided written informed consent. The study is registered on ClinicalTrials.gov under the identifier NCT01743274.
OCT AND FFR ACQUISITION
OCT images were acquired using the FD-OCT OPTIS™ system (Abbott Vascular, Santa Clara, CA, USA) and a 6 Fr guide catheter compatible Dragonfly™ DUO and Dragonfly™ OPTIS™ catheter (Abbott Vascular). FFR was measured using a pressure wire (Abbott Vascular) equipped with a pressure sensor located 30 mm from the distal end of the catheter. Both procedures have been described extensively elsewhere6.
OCT ANALYSIS
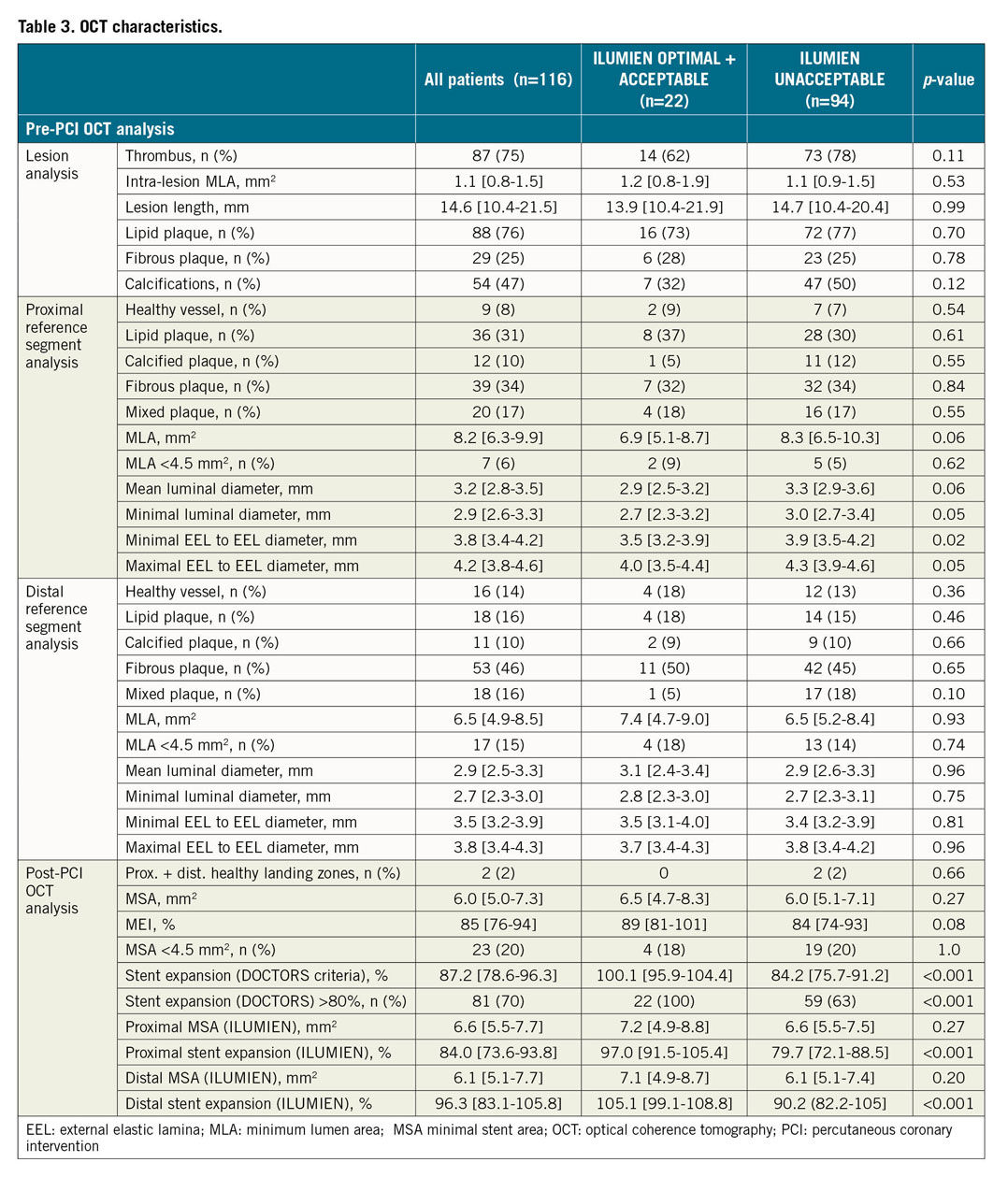
OCT images were analysed offline using the AptiVue™ dedicated manufacturer software (Abbott Vascular) in a centralised core laboratory by two independent operators blinded to the clinical, procedural characteristics and final FFR value. The cross-sectional images were sequentially analysed at 1 mm intervals; discordant analyses were resolved by consensus. On the pre-PCI run, lesion length, minimal lesion area, plaque composition, and presence of thrombus were determined according to the consensus documents8,9. On the post-PCI run, proximal and distal reference segments were identified, allowing quantitative measures of minimal lumen diameter and minimal lumen area (MLA), reference lumen diameter and reference lumen area, minimal stent area (MSA) and presence of residual plaque on landing zones. Furthermore, external elastic lamina was identified on proximal and distal reference segments according to the ILUMIEN III method: minimal, maximal and mean diameters were then measured5. Stent expansion was evaluated by both DOCTORS and ILUMIEN III criteria (Supplementary Table 1)5,6. The stent minimum expansion index (MEI) was calculated according to the previously described method10.
ENDPOINTS
The co-primary endpoints were the success of the procedure according to the ILUMIEN III stent expansion criteria (optimal+acceptable result: distal and proximal expansion >90% reference area)5 and according to the DOCTORS expansion criteria (minimum stent area >80% mean reference segment luminal area)7 (Supplementary Table 1). The secondary endpoints included the achievement of MSA ≥4.5 mm2 (which has been proposed as a marker of adequate PCI results by OCT8) and post-PCI FFR value >0.9 (functional procedural success).
STATISTICAL ANALYSIS
Statistical analysis was performed with SPSS, Version 21.0 software (IBM Corp., Armonk, NY, USA). Continuous numerical data are expressed in median ± interquartile range and qualitative data in percent. The differences between the variables were compared by the chi² or Fisher’s exact test for qualitative variables and by the Mann-Whitney U test or Student’s t-test, depending on the distribution of the variables. The relationship between FFR and expansion indices was examined with Spearman’s correlation test. Binary logistic regression analysis was used to test the relationships between inadequate stent deployment incidence and clinically relevant variables. Univariable regression analyses were performed first to test the relationship between outcome and selected parameters. Then all covariates with a p-value of <0.10 were included in the multivariable regression model and backward stepwise elimination was performed to identify independent predictors of the primary endpoint. A p-value of <0.05 indicated a statistically significant difference.
Results
PATIENTS’ BASELINE CHARACTERISTICS
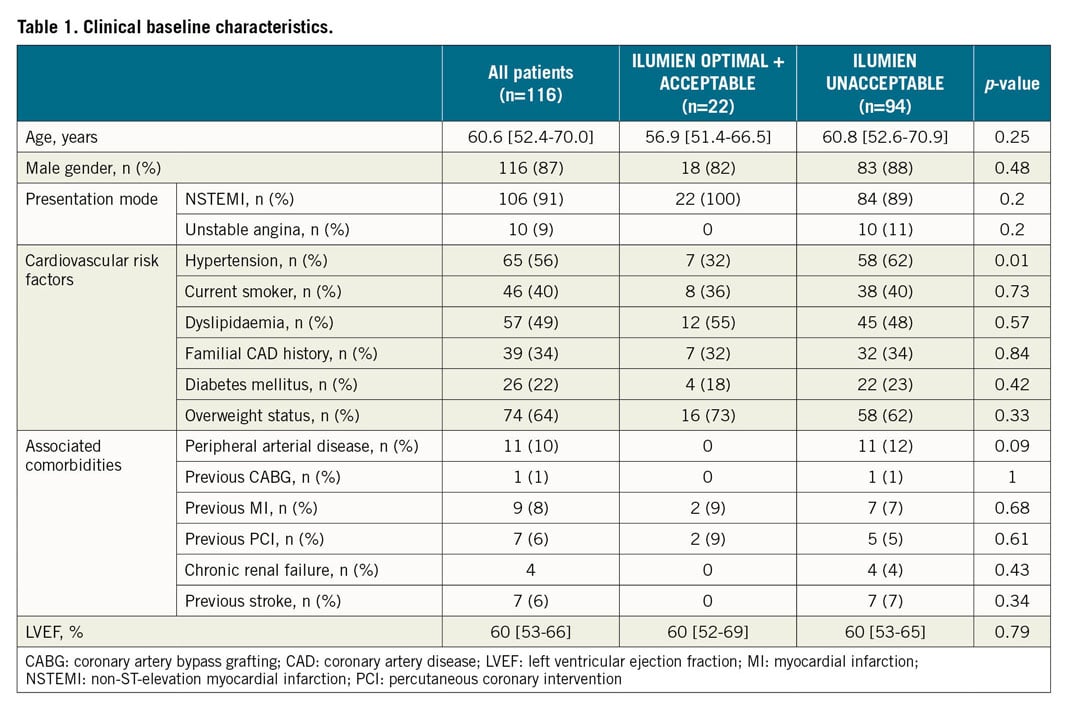
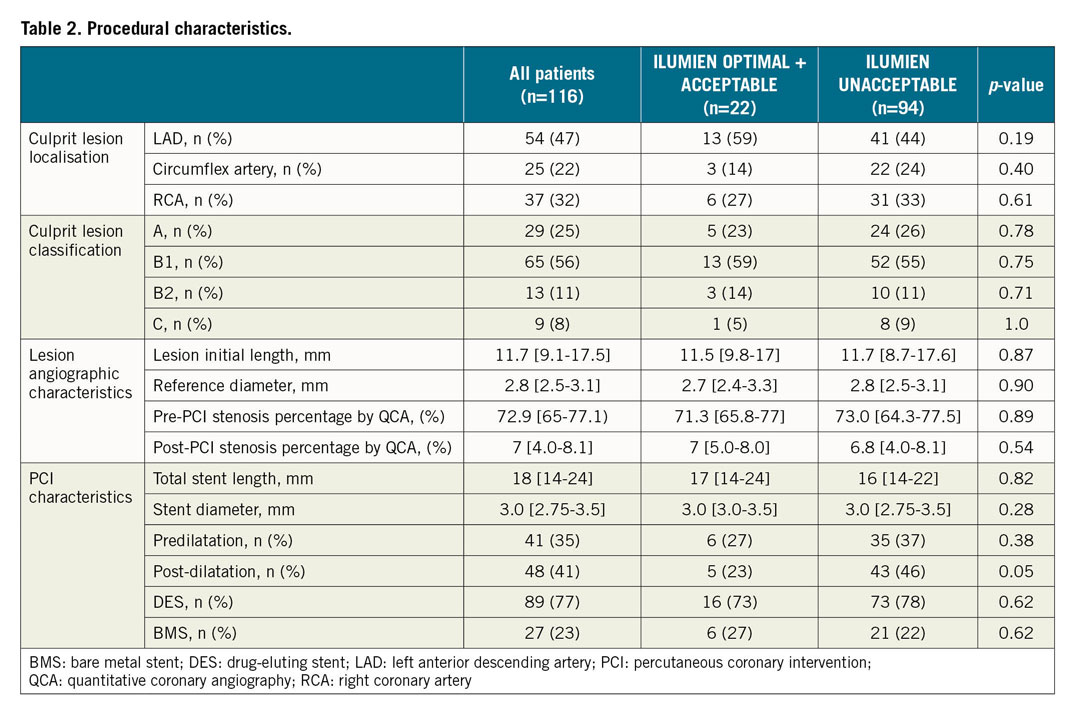
Between September 2013 and December 2015, among 1,935 patients with NSTE-ACS referred for PCI in nine centres, a total of 240 patients were included (120 in the angiography-guided group and 120 in the OCT-guided group). Among these latter, n=116 patients were included in the current analysis and n=4 patients were excluded for poor image quality. The baseline clinical, angiographic and OCT analysis characteristics of these individuals are provided in Table 1-Table 3.
STENT DEPLOYMENT ANALYSIS
Final post-stenting analysis revealed that ILUMIEN III optimal stent deployment was observed in n=12 (10.4%), acceptable deployment in n=10 (8.6%) and unacceptable deployment in n=94 (81%) patients. Furthermore, stent deployment positively met the DOCTORS expansion criteria in n=81 (70%) subjects and the median MEI was 85% (76-94%).
The analysis of the deployment according to the proximal and distal sections of the stent is presented in Figure 1. This deployment was significantly better in the distal compared to the proximal section: the device expansion (ILUMIEN III) was 96.3% (83.1-105.8) in the distal versus 87.2% (78.6-96.3) in the proximal section (p<0.001). Hence, the ILUMIEN III criteria for optimal and acceptable deployment were more frequently obtained in the distal than in the proximal portion (59.5 vs 34.5%, p<0.001). The MSA was not significantly different between the two sections of the stent (6.6 [5.5-7.7] vs 6.1 [5.1-7.7] mm2, p=0.22); however, these results have to be analysed in the light of the smaller values of MLA in the distal compared to the proximal reference arterial segment (6.5 [4.9-8.5] vs 8.2 [6.3-9.9] mm2, p<0.001). Comparable results were also observed for luminal and external elastic lamina (EEL) to EEL diameters (not shown). We also analysed the impact of the stent diameter on the results, by dividing our cohort into patients with implanted device diameter ≤2.75 mm (n=28) and >2.75 mm (n=88). We did not observe any significant difference between the two subgroups for successful deployment according to ILUMIEN III (14.3 vs 20.5%, p=0.47) and DOCTORS criteria (82.1 vs 65.9%, p=0.11).
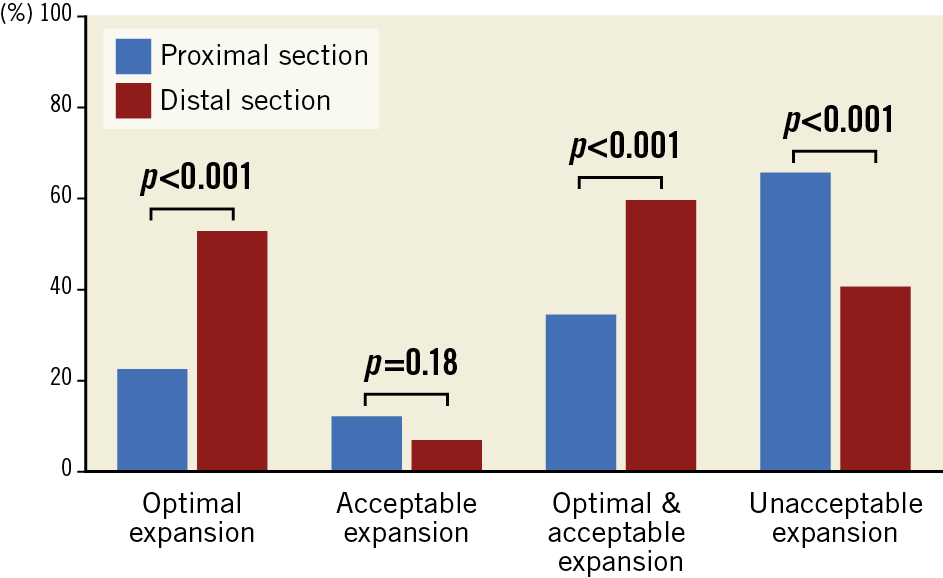
Figure 1. Achievement of optimal, acceptable and unacceptable stent expansion according to ILUMIEN III criteria as a function of the device analysed section (proximal vs distal).
Table 1-Table 3 present the characteristics of the patients with optimal+acceptable final stent deployment compared to the others. Supplementary Table 2 shows the predictors of unacceptable stent deployment in univariable and multivariable logistic regression analyses.
FFR VALUES ACCORDING TO STENT DEPLOYMENT RESULTS
The final post-PCI FFR value in the global cohort was 0.95 (0.92-0.97). There was no difference in the FFR results between the patients with optimal+acceptable stent deployment and the others (0.93 [0.91-0.95] vs 0.95 [0.92-0.97], p=0.22) (Figure 2A). There was no significant difference in the proportion of subjects with final FFR values >0.9 in the two groups - 84% in the unacceptable stent deployment group versus 77% in the optimal+acceptable deployment group (p=0.51). Moreover, we did not observe a significant difference between the FFR values between patients with successful stent deployment according to the DOCTORS criteria compared to the others (0.94 [0.91-0.97] vs 0.95 [0.93-0.97], p=0.23) (Figure 2B) or between patients with MEI ≥74% compared to the others (0.94 [0.92-0.97] vs 0.95 [0.92-0.96], p=0.65) (Figure 2C). Accordingly, there was no significant difference in the proportions of subjects with final FFR values >0.9 between patients with adequate and inadequate stent deployment (respectively, 79% and 91%, p=0.10). Similar findings were observed in patients with stent diameter ≤2.75 mm (not shown). Nevertheless, the final FFR value was significantly higher in patients with final MSA ≥4.5 mm2 (0.95 [0.92-0.97]) than in patients with MSA <4.5 mm2 (0.92 [0.90-0.95], p=0.03) (Figure 2D). There was a correlation between final FFR value and MSA (r=0.188, p=0.04) (Supplementary Figure 1) but not MEI (r=-0.06, p=0.58). Univariable and multivariable logistic regression analysis investigating the predictors of final FFR >0.9 are displayed in Supplementary Table 3.
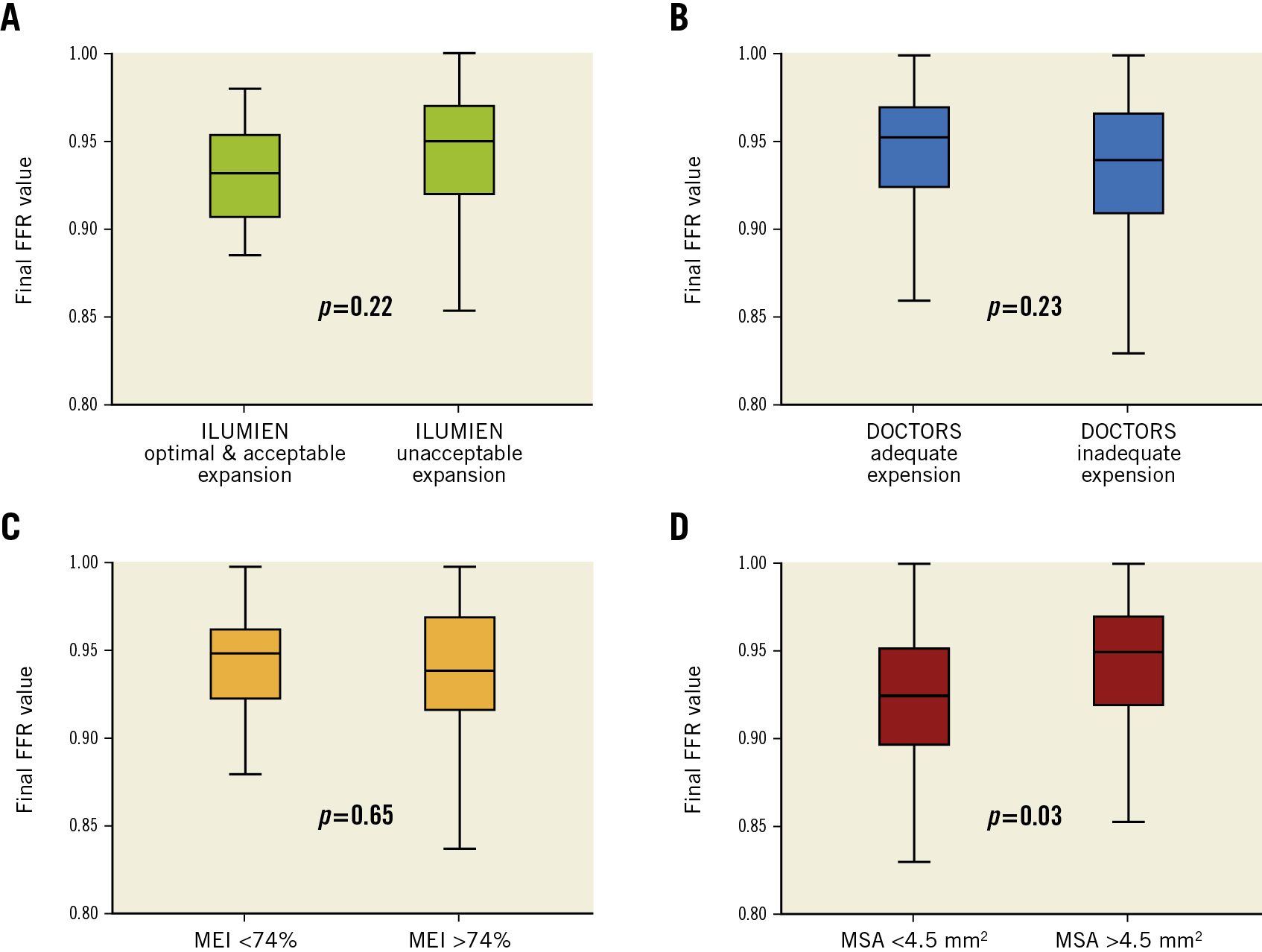
Figure 2. Post-PCI final FFR value according to achievement of optimal stent expansion using ILUMIEN III criteria (A), DOCTORS criteria (B), MEI values (C) or MSA values (D).
Altogether, these results suggested that “optimal” stent deployment (as assessed by geometric ratio) did not significantly affect post-PCI physiological results in this population.
Discussion
The main findings of the current study can be summarised as follows: 1) optimal stent deployment according to the ILUMIEN III criteria was infrequently achieved in this cohort, although being frequent according to DOCTORS criteria; 2) inadequate stent deployment as assessed by geometric ratio did not influence PCI physiological results.
One of the main purposes of PCI optimisation is to achieve full lumen expansion. Stent underexpansion is a major trigger for persistent angina, restenosis, and acute/subacute/late stent thrombosis2,3,4. Previous studies that used IVUS to define and achieve optimal stent expansion have reported contrasting success rates for clinical outcome improvement, depending on the lesion location2,10. OCT, with its higher-resolution imaging capability and semi-automated image analysis, could appear to be an adequate tool to guide optimal expansion. Hence, OCT guidance has been reported to achieve comparable degrees of stent expansion to those with IVUS guidance11,12. However, very few data are available regarding the best OCT criteria to use. Whether achieving pre-specified “optimal” expansion could improve prognosis is unknown. Most of the proposed criteria rely on the use of cross-sectional analysis and conventional geometric methods to compare the minimal area of a single frame (within the stent) with the reference vessel dimensions. The OPINION trial proposed that adequate stent deployment would be defined as an MSA value ≥90% of the average reference lumen area13. The DOCTORS study decreased the cut-off value for MSA to 80% of the average lumen area6. More recently, the ILUMIEN III investigators proposed a new methodology based on the device segmentation in two sections and increased the MSA cut-off value to 95% of the reference area. In this OCT-guided PCI series, we observed that 70% of the patients met the DOCTORS adequate expansion criteria, whereas only 19% presented optimal or acceptable deployment according to ILUMIEN III standards. This major discrepancy might have several explanations. First, the achievement of the ILUMIEN III criteria was not a predefined endpoint in the DOCTORS trial. Second, obtaining stent expansion according to these latter criteria is challenging. Hence, in the ILUMIEN III trial, 59% and 63% of the patients from the OCT guidance and IVUS guidance groups, respectively, were unable to achieve these expansion objectives. Whereas intensive PCI optimisation strategies were used by the investigators in both studies (including an intensive post-dilation strategy), these data show that optimal geometric expansion could not be obtained in a significant percentage of patients. Moreover, this “aggressive” approach could also result in acute complications (dissections, coronary artery perforations or haematoma) and lead to longer procedure time. Finally, one should also bear in mind that the two studies included different profiles of patients: whereas ACS represented 100% of the patients included in DOCTORS, unstable angina and NSTEMI accounted for roughly one third of the cases included in ILUMIEN III5,6. These differences in baseline characteristics and, potentially, in lesion composition, could have influenced the final results.
An impaired post-PCI FFR value is associated with increased major adverse cardiovascular events in patients with either ACS or stable coronary artery disease (CAD)14. Inadequate stent expansion is frequently suspected to induce suboptimal post-PCI FFR results15. Hence, applying post-dilation could increase the FFR values16, especially when intracoronary imaging guidance is provided17,18. However, in the current series, we did not observe any impact of inadequate stent expansion, assessed by ILUMIEN III and DOCTORS criteria, on final PCI physiological results. These results are in line with previous observations from Nakamura et al who failed to observe any comparable association between FFR and conventional geometric stent expansion measurement methods10. Several hypotheses could explain these findings. First, other elements could impair post-PCI FFR values such as an associated second lesion, residual diffuse disease, geographical gaps, edge dissections or pressure sensor drift15. Second, the calculation of stent expansion according to the ILUMIEN III and DOCTORS criteria is based on conventional geometric methods. This approach might be inappropriate in several situations, including bifurcations or tapered vessels, leading to underestimation of correct device expansion. Thus, alternative and more accurate methods are required. Nakamura et al recently proposed an OCT-derived volumetric stent expansion analysis leading to the calculation of a minimum expansion index that was correlated to post-PCI FFR values in stable patients10; however, we did not observe comparable results in the DOCTORS cohort. The crude MSA value could also be used: the recent EAPCI expert consensus document proposed using MSA ≥4.5 mm2 by OCT as a cut-off point to distinguish acceptable and non-acceptable stent expansion8. Interestingly, in the current series, we observed significantly higher values of FFR in patients with MSA ≥4.5 mm2 than in patients with MSA <4.5 mm2, suggesting the potential relevance of this strategy.
Limitations
Although reporting the results from one of the largest series of patients explored by both OCT and FFR, the current study has some limitations. First, all the patients included in the DOCTORS study suffered from NSTE-ACS, thus the translation of our results in subjects with stable CAD has to be proven. Second, the patients included in the current analysis represent a selected population, with a high percentage of post-dilation, and were considered to have successful OCT-guided PCI and acceptable stent expansion according to the protocol. Thus, we did not include patients with severe stent underexpansion (MSA <60% average reference segment area) and are not able to establish its impact on procedural physiological efficiency, which might have impacted on the correlations between expansion indices and FFR values. Third, the median stent length in this cohort was somewhat short and we do not know if the results would be comparable with longer devices, where the incidence of inadequate expansion is higher. Finally, the current analysis only investigated the relationships between FFR values and device expansion assessed by intracoronary imaging, as this was the primary endpoint of the DOCTORS study. Thus, the impact of inadequate stent expansion according to geometric criteria or MEI on clinical outcome remains to be determined in this cohort.
Conclusions
In the current analysis that included selected patients with OCT-guided PCI and substantial use of post-dilation, we did not observe any impact of optimal stent expansion according to ILUMIEN III or DOCTORS OCT criteria on final post-PCI FFR results. These data confirm that post-PCI FFR values are influenced by multiple parameters.
|
Impact on daily practice The best criteria for adequate stent expansion assessment by intracoronary imaging remain debated and their correlation with post-PCI FFR value is unknown. This study shows that the optimal stent expansion according to the ILUMIEN III or DOCTORS criteria is poorly related to post-PCI FFR value. Conventional geometric methods for assessing adequate device deployment might be limited and new imaging strategies are needed to predict PCI functional success better. |
Funding
The DOCTORS study was funded by the French government’s national hospital research programme (Programme Hospitalier de Recherche Clinique 2013).
Conflict of interest statement
N. Meneveau declares consulting fees and speaker honoraria (modest) from Abbott Medical, Bayer, Daiichi Sankyo, AstraZeneca, BMS-Pfizer, and speaker honoraria from Boehringer Ingelheim. P. Motreff declares consulting fees (modest) from Terumo and Abbott Medical. N. Amabile declares research grants to the institution from Abbott Medical and consulting fees (modest) from Abbott Medical and Boston Scientific. J. Silvain declares consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, CSL Behring, Gilead Science and Sanofi-Aventis, speaker honoraria from AstraZeneca, Amgen, Bayer, Algorythm and Sanofi-Aventis, and travel support from Amgen, AstraZeneca, Bayer and Bristol-Myers Squibb. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

