Stent underexpansion is a major contributing factor to stent failure, be that restenosis or stent thrombosis. Intracoronary imaging is increasingly being used to optimise the results of percutaneous coronary intervention (PCI) and has been shown to decrease the rate of underexpansion. However, optimal imaging-defined expansion criteria vary across studies. Furthermore, the correlation between these expansion criteria and the physiological results of stenting have not been extensively investigated.
In this issue of EuroIntervention, Belguidoum and colleagues present a post hoc analysis of the optical coherence tomography (OCT) cohort of the DOCTORS study1.
The DOCTORS study randomised non-ST-elevation acute coronary syndrome (ACS) patients to OCT-guided versus angiography-guided PCI and compared the post-PCI fractional flow reserve (FFR) results between groups, finding improved FFR in the OCT group2. The current substudy evaluates the OCT arm applying differing expansion criteria3 and comparing the final FFR results between those with acceptable/optimal expansion versus those with inadequate expansion as per these criteria. The main findings of the study are the following. 1) A much lower proportion of patients achieved optimal expansion according to ILUMIEN III criteria as compared with DOCTORS criteria. 2) There was no relationship between post-PCI FFR values and optimal stent expansion.
Differences in the definition of optimal stent expansion between DOCTORS and ILUMIEN III (Figure 1) can explain the discordance in results obtained using the two methods2,3. The higher proportion of suboptimal results in terms of expansion when applying the ILUMIEN III criteria can have several explanations.
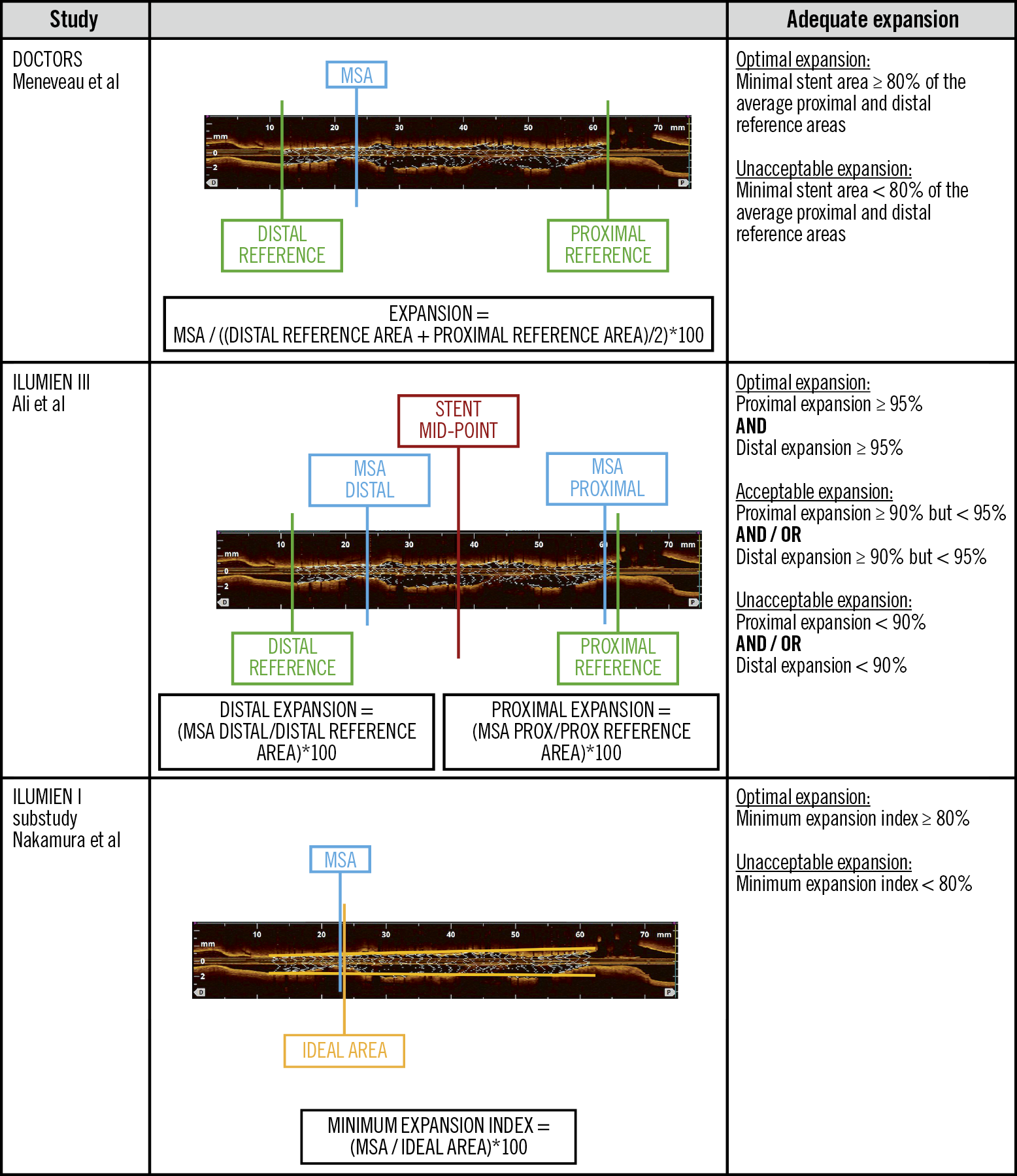
Figure 1. Stent expansion criteria. The figure shows 3 different methods to evaluate stent expansion.
Optimal stent expansion depends on a number of factors, including plaque preparation, correct stent sizing and appropriate post-dilation with balloons adjusted to the vessel size, which is particularly important for long stents in tapering vessels.
The inability to obtain an optimal expansion according to the more strict ILUMIEN III criteria could be associated with inadequate plaque preparation (in the context of ACS where plaque preparation is usually less aggressive due to the risk of embolisation). Predilation was performed in 35% overall, with no differences between those with and those without acceptable expansion by ILUMIEN III criteria. The presence of calcification in the optimal/acceptable and unacceptable groups was similar; however, a very limited analysis of calcium was provided in the study.
Post-dilation was performed in only 41% overall (48/116 patients) and more frequently in patients with non-optimal stent expansion according to the ILUMIEN criteria (46% vs 21% in the optimal group). The low rates of both predilation and post-dilation may be related to the types of patient evaluated in this study (ACS with thrombus in 75%). It is likely that those achieving optimal/acceptable expansion as per the ILUMIEN III criteria represent a group of patients with very soft, non-calcified lesions that did not require further optimisation after nominal stent implantation. Therefore, optimal stent expansion was related more to the characteristics of the underlying lesion being treated, rather than to the use of stent optimisation techniques. However, 81% did not achieve optimal expansion by ILUMIEN III criteria (30% by the DOCTORS study protocol criteria), which is surprising in this ACS population with 76% having lipid-rich plaques by OCT.
Another interesting finding in this study is the degree of stent undersizing according to ILUMIEN III criteria. The minimal external elastic lamina (EEL) to EEL diameter in the distal reference was 3.5 mm; however, the mean stent diameter used in the present study was 3.0 mm. In the main DOCTORS study, it is mentioned that the diameter of the stent to be implanted was to be chosen based on the quantitative measures of reference vessel diameter by OCT. However, it is not specified which criteria were used (lumen or EEL diameter) to select the stent size. This might explain the discordance observed and indicate that probably in many cases, especially when there were no healthy landing zones, the lumen was used for stent sizing. Further, in the main DOCTORS study there was no difference in the stent diameter between the angiography- and OCT-guided groups when many studies have demonstrated that using intracoronary imaging leads to the use of larger stents in terms of diameter and length4. This again points to the use of a conservative sizing strategy in the OCT arm, reinforced by a resulting minimal stent area (MSA) of <4.5 mm2 in one fifth of patients despite mean vessel reference values of 3.5 mm2. Again, the study population must be considered here: many ACS patients present with significant coronary spasm with thrombus complicating the measurement of vessel size using intracoronary imaging, resulting in undersizing.
A larger proximal reference area was a predictor of inadequate stent expansion. This might reflect the inaccuracy of the methods evaluated to calculate stent expansion when there is vessel tapering. However, suboptimal expansion was generally in the proximal stent segment which may again reflect stent undersizing and failure to adjust post-dilation balloon sizes to the vessel size in the context of vessel tapering. This is reinforced by the lack of difference in the MSA between the distal and proximal stent segments, indicating that the size of the post-dilatation balloon was probably not adjusted to the proximal vessel size. It is, however, interesting that, in general, the stents were short (mean 18 mm, range 14-24 mm). With such short stents it is difficult to understand that there would be much vessel tapering to justify the suboptimal stent expansion in the proximal segment. On the other hand, the vessel most frequently evaluated was the left anterior descending (LAD), which is known to have more vessel tapering due to its multiple side branches.
Several factors can influence the physiological result after stenting, including not only stent underexpansion but also geographical miss or presence of other lesions in the vessel. The DEFINE PCI study evaluated the rate of residual ischaemia after PCI and found abnormal instantaneous wave-free ratio (iFR) values after PCI in 24% of 500 patients assessed5. The pressure pullbacks demonstrated that most physiologic abnormalities after PCI were related to focal stenosis located proximal or distal to the stent. It is therefore complex to establish a relation between stent expansion and FFR unless there is just a single lesion to be treated without residual disease. The present study does not provide information about these findings as physiologic evaluation did not include pullbacks and there is no information about the presence of other lesions in the vessel. It is difficult therefore to draw conclusions from the lack of statistical difference between FFR values in optimally expanded stents and those with unacceptable expansion.
The other important problem in attempting to establish a relationship between the post-PCI FFR and stent expansion in the current study is the type of lesions included (culprit vessel in non-ST-segment myocardial infarction [NSTEMI] patients). Microcirculatory dysfunction often present in the context of ACS can impair the ability to achieve maximal hyperaemia using adenosine (the hyperaemic agent used in this study), leading to higher FFR values6. It is therefore very difficult to clarify the influence of the stent expansion alone on the FFR value obtained in these vessels. It would have been very informative for the DOCTORS study to determine the FFR pre-intervention and to assess the physiological changes after stenting.
A previous study by Nakamura et al was able to establish an association between post-PCI FFR and stent expansion assessed as minimum expansion index (MEI) with the use of a novel volumetric algorithm in a population of stable patients7 (Figure 1). In the current analysis, the authors did not find evidence of a relationship between MEI and FFR. The differences in the type of population evaluated and indices used to assess expansion can explain this discordance. This highlights the complex relation between the geometric and functional results of PCI.
It is true that the ILUMIEN III criteria for optimisation can be difficult to achieve and should be adapted when there is vessel tapering to avoid procedural complications such as perforations in relation to the use of oversized balloons. However, the results of this substudy point towards a lack of stent optimisation in this population, regardless of the OCT criteria used. This might not influence the immediate result of FFR but could potentially influence the long-term performance of the stent. This will be clarified with the results of ILUMIEN IV, a randomised study comparing angiography and OCT guidance for stent optimisation powered for clinical endpoints.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

