
Abstract
Aims: Fractional flow reserve (FFR) can be used to detect a suboptimal result after percutaneous coronary intervention (PCI). A lower post-procedure FFR (<0.90) has been shown to be associated with adverse clinical outcomes at follow-up. This pilot study aimed to understand the mechanisms resulting in a suboptimal FFR and whether optical coherence tomography (OCT)-guided optimisation could improve final FFR.
Methods and results: Thirty-five patients undergoing complex PCI were prospectively enrolled. After stenting and post-dilatation, OCT and pressure wire were performed. An FFR threshold <0.90 after PCI was defined as suboptimal and mandated further PCI optimisation. A satisfactory post-PCI FFR (predefined as ≥0.90) was achieved immediately after conventional PCI in 14 patients (40%) and in this group no further treatment was performed. Minor abnormalities (stent malapposition of 200-500 µm) were observed with OCT in three of these patients. Suboptimal functional results after conventional stenting (predefined as an FFR <0.90) were found in 21 patients (60%). In thirteen out of these 21 patients (61.9%), OCT demonstrated a suboptimal stent result. Subsequent OCT-guided optimisation was performed resulting in a higher final FFR (increase from 0.80±0.02 to 0.88±0.01; p=0.008).
Conclusions: Despite a satisfactory angiographic result, a suboptimal functional result is evident in a substantial proportion of patients undergoing complex PCI. Implementation of an OCT-guided PCI optimisation protocol may reveal potentially treatable causes, allowing optimisation of the post-PCI functional result.
Abbreviations
FFR: fractional flow reserve
MACE: major adverse cardiovascular events
NSTE-ACS: non-ST-segment elevation acute coronary syndromes
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
POBA: plain old balloon angioplasty
Introduction
FFR measured in the catheterisation laboratory is a widely accepted method of assessing ischaemia, with FFR measured prior to PCI having a Class IA indication in the European guidelines1. Although angiography is an accurate way of assessing the safety of the initial result of stent deployment, it has limited efficacy to predict the physiological result of treatment and subsequent clinical outcome2,3.
In the FAME study, patients with a satisfactory angiographic result had a 20% rate of major adverse cardiovascular events (MACE) at two years4,5. Subsequent analysis of the final functional result after PCI demonstrated that a lower post-PCI FFR was closely associated with a worse vessel-related outcome. These data have been confirmed by summated data demonstrating a close relationship between impaired post-PCI FFR and poor clinical outcome in other trials6-16. A threshold of final FFR <0.90 has been proposed to define a suboptimal result after stenting8,11,12,15.
Two recent studies have suggested that additional intervention may optimise the outcome in patients with suboptimal FFR14,16. However, the method by which post-PCI optimisation was performed was not standardised in these studies and the rate of intravascular imaging guidance was low (<10% of cases). OCT is regarded as the gold standard for imaging acute stent deployment. A recent study suggested that suboptimal stent deployment may occur in up to 30% of cases and is linked to a poor clinical outcome17. The DOCTORS study by Meneveau et al showed that PCI guided by OCT resulted in a higher post-PCI FFR compared to angiography alone in patients with NSTE-ACS18. Notably, these investigators performed OCT-guided optimisation in every patient irrespective of whether initial stenting had a functional result that was satisfactory.
This pilot study aims to investigate the incidence of a suboptimal FFR in a consecutive cohort of patients undergoing complex PCI and to assess the impact of an algorithm based on combined FFR/OCT measurements to guide PCI optimisation.
Methods
The OxOPT-PCI study is a prospective, observational, single-centre trial, conducted at the Oxford Heart Centre, Oxford University NHS Foundation Trust. The protocol was approved by the local ethics committee (REC number 10/H0408/24) and conducted in accordance with the Declaration of Helsinki. A preliminary analysis of the data was mandated to assess the efficacy and safety of the approach.
PATIENT POPULATION
Eligible subjects were referred for elective or urgent complex PCI between September 2016 and July 2017 at the John Radcliffe Hospital, Oxford Heart Centre, United Kingdom. The following inclusion criteria were used: (i) age between 30 and 90 years, (ii) angiogram showing an angiographically severe lesion, suitable for both PCI and intravascular OCT. The following exclusion criteria were applied: (i) ST-elevation myocardial infarction; (ii) revascularisation by balloon angioplasty without stenting; (iii) known severe renal failure (estimated glomerular filtration rate [eGFR] <30 ml-1 min-1 1.73 m2), history of dialysis, or renal transplant; (iv) presentation with cardiogenic shock; (v) contraindications to adenosine.
STUDY PROTOCOL
Figure 1 shows the study flow chart. All patients underwent conventional angiography-guided stent deployment, until an acceptable angiographic result was achieved (all treatment decisions at this stage were left to the operator’s discretion).
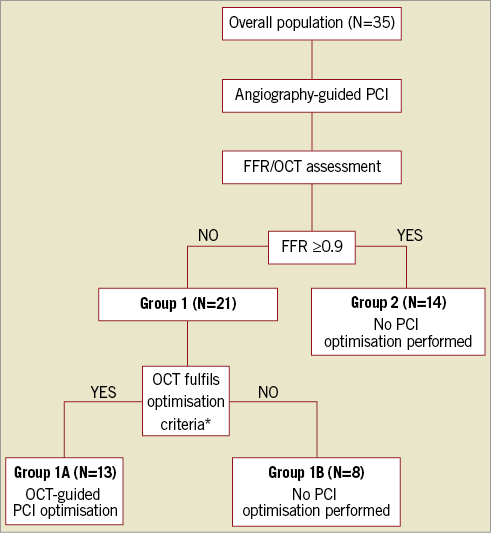
Figure 1. Flow chart of the study population and design. *Presence of one or more of the following according to CLI-OPCI study criteria17: stent underexpansion and/or incomplete lesion coverage and/or stent malapposition and/or stent-edge dissection and/or intra-stent plaque/thrombus protrusion. FFR: fractional flow reserve; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Following completion of conventional PCI, FFR was measured, and intracoronary OCT imaging was performed. The study population was then divided into two groups based on the observed FFR value: Group 1 patients with a suboptimal functional result (FFR <0.90); Group 2 patients with a satisfactory functional result (FFR ≥0.90). For patients in Group 2, no further PCI optimisation was performed and OCT acquisition was performed only as a safety measure, to assess the frequency of relevant vessel-related complications (Supplementary Appendix).
In patients in Group 1, OCT was performed per protocol after achievement of satisfactory angiographic results. OCT was analysed according to the CLI-OPCI study defining a suboptimal stent result according to specific criteria (Supplementary Appendix)17. Operators were mandated to perform PCI optimisation according to the respective OCT finding (Supplementary Appendix).
After the optimisation procedure, FFR and OCT assessment were repeated. If post-optimisation FFR was ≥0.90, procedure and protocol were both considered complete. If post-optimisation FFR was still <0.90, optimisation was repeated. Optimisation was limited to two attempts, unless further optimisation was felt to be clinically indicated by the treating operator.
For patients in Group 1 who did not fulfil the OCT criteria (Group 1B), further PCI optimisation was not performed.
FRACTIONAL FLOW RESERVE MEASUREMENT
FFR was measured before stenting, after conventional stenting, and following each PCI optimisation attempt. A coronary pressure guidewire (St. Jude Medical, St. Paul, MN, USA) was first balanced and, after controlling for pressure normalisation, it was advanced distal to the lesion. After administration of intracoronary nitrates, FFR was measured under maximal hyperaemia, using intravenous adenosine (140 mg kg-1 min-1), as the ratio between distal coronary pressure (Pd) and aortic pressure (Pa). After stenting, in case of suboptimal FFR (<0.90), manual pullback of the pressure wire was performed during maximal hyperaemia to localise any area of pressure change. Diffuse coronary artery disease was suspected as the underlying cause of a suboptimal post-PCI FFR if a continuous gradual increase in FFR without clear step-up occurred during pullback of the pressure wire along the course of the coronary artery. In the presence of a significant pressure step-up the location of the pressure wire was matched with the coronary angiogram. Subsequently, anatomical landmarks were identified on the angiogram (e.g., side branches, calcific nodules) and exactly matched with the corresponding OCT in order to identify the area relating to the suboptimal FFR.
OPTICAL COHERENCE TOMOGRAPHY IMAGE ACQUISITION
Optical coherence tomography analysis (ILUMIEN™ OPTIS™ system - Dragonfly™ Duo Imaging Catheter; St. Jude Medical) was performed after conventional stenting and following each PCI optimisation procedure. OCT imaging was performed on a 54 mm length segment, using a non-occlusive technique with automated pullback at 18 mm s-1 during manual intracoronary injection of iso-osmolar contrast. Notably, FFR measurement and OCT imaging were both performed at the same point in the procedural sequence.
The OCT criteria used to determine whether PCI optimisation was applicable were based on the CLI-OPCI study17 (Figure 2, Supplementary Appendix): i) stent underexpansion; ii) incomplete lesion coverage; iii) stent malapposition; iv) stent-edge dissection, and v) intra-stent plaque/thrombus protrusion.
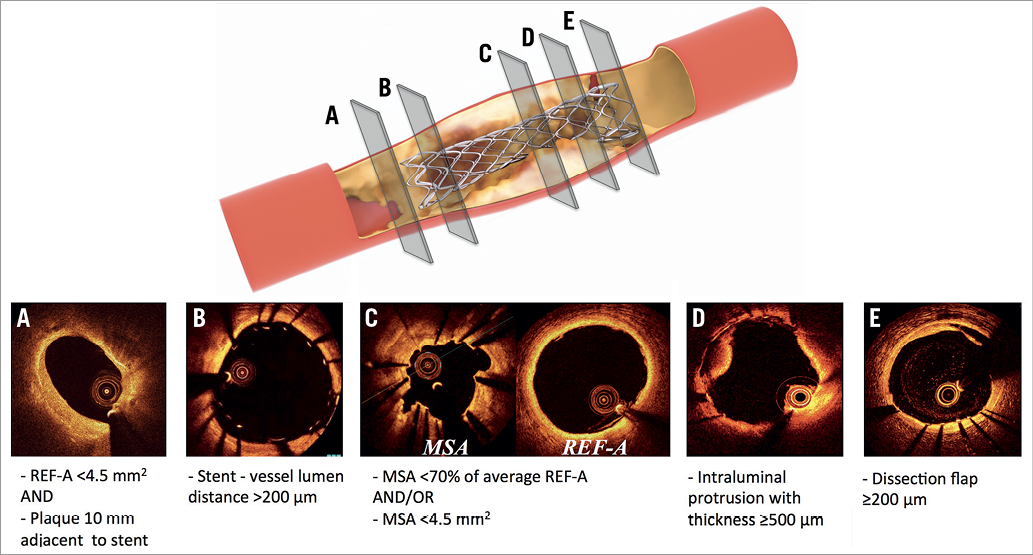
Figure 2. Definitions of OCT criteria for suboptimal stent deployment. A) Incomplete lesion coverage. B) Stent malapposition. C) Stent underexpansion. D) Intra-stent plaque/thrombus protrusion. E) Edge dissection. FFR: fractional flow reserve; MSA: minimal stent area; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; REF-A: reference lumen area. Adapted from Wolfrum et al15 under the terms of the Creative Commons Attribution 4.0 International License.
QUANTITATIVE CORONARY ANGIOGRAPHY
Angiograms were analysed at three different time points: pre-PCI, following conventional PCI, and following final PCI optimisation, if performed. Angiograms were analysed with two-dimensional quantitative coronary angiography (QCA) by two experienced operators (M. Wolfrum, S. Benenati) using dedicated software (Medcon QCA software; Medcon Limited, Tel Aviv, Israel) with an automated edge-detection algorithm. Discordant analyses were resolved by consensus with a third operator (G.L. De Maria), also blinded to FFR and OCT data.
STATISTICAL ANALYSIS
All continuous variables are expressed as mean and standard deviation (±SD) or as median of the respective interquartile range (IQR) as appropriate after controlling for normal by Shapiro-Wilk test. Frequencies were expressed as percentage and compared using the chi-square test or Fisher’s exact test as appropriate. Comparisons between continuous variables were performed by t-test or ANOVA, if normally distributed. Mann-Whitney or Kruskal-Wallis tests were used to compare non-normally distributed variables. P-values <0.05 were considered statistically significant. Statistical analyses were performed with SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
STUDY POPULATION
Baseline characteristics are presented in Table 1. Notably, two thirds of enrolled patients presented with stable angina and one third with non-ST-segment elevation acute coronary syndromes (NSTE-ACS). The majority of patients (86%) had complex single-vessel disease (69% with B2 or C ACC/AHA lesion type), primarily involving the left anterior descending artery (LAD, 71%).
ANGIOGRAPHIC CHARACTERISTICS AND PROCEDURAL STRATEGY FOR CONVENTIONAL STENTING
Before stenting, percent diameter stenosis was 63.7±15.4% and the FFR was 0.58±0.14 (Table 2, Supplementary Table 1). Eighty percent (80%) of patients underwent predilatation before stenting (Table 3). On average 1.4 stents were deployed. Post-dilatation was performed in 91% of patients during conventional stenting (no statistical difference among the study groups defined below). Table 3 depicts in detail the strategy utilised during conventional stenting. After conventional stenting, a satisfactory angiographic result was achieved which was reflected by a residual diameter stenosis of 5.8±12.1%. Repeat FFR after PCI showed on average an effective ischaemia reduction from 0.58±0.14 pre-stenting to 0.80±0.02 after conventional stenting (p=0.001).

DISTRIBUTION OF STUDY GROUPS ACCORDING TO HAEMODYNAMIC AND OCT RESULTS AFTER CONVENTIONAL STENTING
Group 1 included 21 patients (60%) with a functionally suboptimal PCI result (FFR <0.90) (Figure 1, Figure 3A). Of these, 13 also had OCT results requiring protocol-mandated OCT-guided PCI optimisation (Group 1A) (Figure 1, Figure 3). In eight patients (Group 1B), PCI optimisation was not performed because none of the CLI-OPCI OCT criteria was fulfilled defining the appearance of a suboptimal stent result (Figure 3A). In all of these Group 1B patients there was angiographic/OCT evidence of diffuse distal disease with no significant pressure step-up during pressure wire pullback.
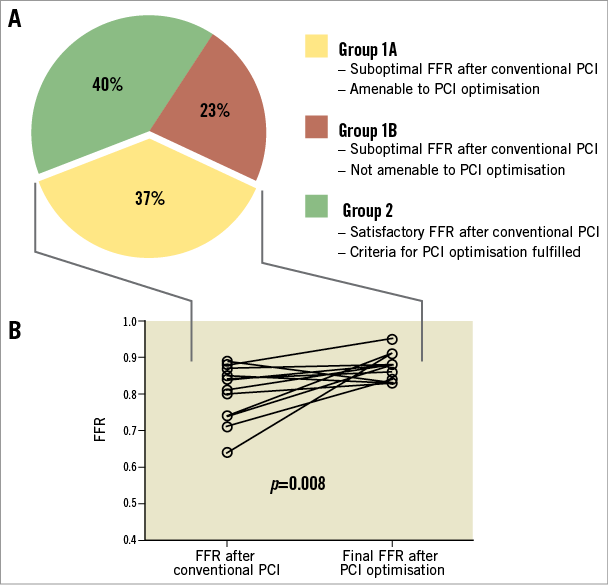
Figure 3. Distribution of study population and impact of PCI optimisation on final FFR. The graph in panel A describes the distribution of the study population according to FFR after conventional PCI. The line graph in panel B depicts the evolution of FFR after PCI optimisation for each individual patient in Group 1A. FFR: fractional flow reserve; PCI: percutaneous coronary intervention
Fourteen patients presented a satisfactory post-stenting FFR ≥0.90 after conventional PCI (Group 2). Notably, in these patients only minor stent-related complications were observed in three patients on OCT imaging (Supplementary Table 2). These were minor stent malapposition, minor distal stent-edge dissection and one case of minor tissue protrusion.
IMPACT OF OCT-GUIDED PCI OPTIMISATION ON POST-PCI FFR
Patients in Group 1A had a significant pressure step-up during pressure wire pullback and suboptimal post-stenting OCT results. Stent optimisation post-dilatation sizing was guided by OCT, resulting in the selection of larger balloon diameters compared to the post-dilatation balloons used during conventional stenting (0.33±0.64 mm, p=0.09) (Table 3). Subsequently, stent expansion in Group 1A patients increased on average from 69% immediately after stent implantation to 86% after optimisation (p=0.001) (Table 2).
The primary reasons for post-PCI optimisation were stent underexpansion and stent malapposition, followed by incomplete lesion coverage, and edge dissection (Supplementary Table 3). Five patients in Group 1A (38.5%) showed both stent underexpansion and stent malapposition. As mandated by the study protocol, post-dilatation was performed in all patients with stent underexpansion and stent malapposition (Supplementary Table 3). Additional stent implantation was performed in seven patients (five for incomplete lesion coverage and two for edge dissection). In Group 1A patients, OCT-guided optimisation was associated with a significant improvement in FFR from 0.80±0.02 to 0.88±0.01 (p=0.008) (Table 2, Supplementary Table 4, Figure 3B).
Discussion
In this preliminary study we demonstrate that a substantial number of patients (~60%) undergoing complex PCI have a suboptimal functional result after conventional PCI (FFR <0.90), despite a satisfactory angiographic result. However, using OCT-guided PCI optimisation in patients with evidence of both suboptimal FFR and OCT resulted in improvement in the final FFR. OCT imaging in patients with post-stenting FFR ≥0.90 did not identify significant abnormalities that required additional interventions.
In our cohort we used an FFR threshold of 0.90 to differentiate patients with a suboptimal PCI result. Although cut-off FFR values ranging from 0.86 to 0.96 were suggested in previous studies to predict future clinical outcome10,13,14, a value of 0.90 was supported by other studies8,11,12 and meta-analysis15. In the present study, patients with post-PCI ≥0.90 did not undergo further PCI optimisation, and OCT analysis showed that no major abnormality in stent deployment was missed.
POOR FUNCTIONAL PCI RESULT DESPITE A SATISFACTORY ANGIOGRAPHIC RESULT
In this and other series, 60% of patients had an unsatisfactory functional result after conventional PCI13,16,18. A number of factors can result in persistence of the pressure gradient after stenting in a diseased epicardial segment. These include incomplete stent expansion, stent malapposition, incomplete lesion coverage, plaque protrusion, and stent-edge dissection (Figure 2)15. This correlation between a persistent pressure gradient and a suboptimal PCI result has been consistently reported6-12,14. Notably, intravascular imaging studies have shown that OCT-defined suboptimal stent results are also associated with poor clinical outcome and predictably many of the same factors are responsible for the poor clinical outcome including stent underexpansion, stent-edge dissection, and incomplete lesion coverage17.
In our study, factors reflecting suboptimal stent deployment were predominantly found in patients in Group 1A, who also had an impaired FFR after conventional stenting. Stent underexpansion and incomplete lesion coverage were the main OCT findings associated with suboptimal FFR. Change in FFR after further treatment was primarily attributable to increased stent expansion and improved coverage of residual coronary plaque disease. The value of OCT in identifying and correcting these pathologies was demonstrated in the ILUMIEN III study19. A significantly lower rate of stent-related complications such as dissections and major stent malapposition was found in the OCT-guided PCI group compared to the IVUS- or angiography-guided PCI group. Furthermore, this study demonstrated that external elastic lamina-based OCT sizing is safe and effective in achieving similar stent expansions compared with IVUS and superior stent expansion compared with angiography guidance.
This preliminary study is the first to apply an FFR/OCT-guided optimisation algorithm prospectively, mandating a specific intervention for each cause of suboptimal stent deployment. Although the study by Agarwal et al showed that additional intervention in patients with a post-PCI FFR in the ischaemic range (≤0.81) led to an overall increase in FFR16, the optimisation strategy was left to the discretion of the operator with some form of intravascular imaging undertaken in only 9% of the cases.
In the DOCTORS study, Meneveau et al showed that OCT-guided PCI is associated with higher post-PCI FFR than PCI guided by angiography alone in patients with NSTE-ACS18. Notably, these investigators performed OCT-guided optimisation in every patient irrespective of whether initial stenting had a functional result that was satisfactory. In contrast, we used post-PCI FFR as an initial stratification tool to identify patients who did not require further stent optimisation, ultimately reducing procedure time, contrast media and radiation.
In patients with an impaired post-PCI FFR, but with no OCT evidence of stent-related complications, OCT-guided PCI optimisation was also deferred. Notably, all of these patients showed no clear step-up in FFR along the coronary artery during pressure wire pullback, a finding consistent with diffuse atherosclerosis. Even though confirmation should come from larger randomised clinical studies, it is reasonable to speculate that additional management strategies may be required rather than further PCI optimisation for diffuse coronary artery disease (CAD).
The link between isolated stent malapposition and reduced post-PCI FFR is speculative, especially when drug-eluting stents with thin struts are used (strut thickness of stents used in our trial: ~80-90 μm). Notably, in our study stent malapposition and an abnormal final FFR were not isolated findings and only occurred together with stent underexpansion and/or incomplete lesion coverage. Consequently, these issues represent the mechanism behind unsatisfactory functional PCI results rather than malapposition per se14,16,18. This may also explain why several previous IVUS and OCT studies have failed to establish a link between acute stent malapposition and clinical outcome at follow-up17,20-22.
Notably, in two patients of Group 1A isolated distal stent-edge dissection was found on OCT during PCI optimisation and subsequently treated with a stent. In both patients the FFR was significantly improved after PCI optimisation. This gives some mechanistic insight into the fact that stent-edge dissection can cause an impaired functional PCI result, which can potentially translate into an adverse clinical outcome, as demonstrated by the CLI-OPCI study for distal stent-edge dissections (HR 4.65, 95% CI: 2.5-8.8)17.
Several trials investigating the importance of coronary imaging-based PCI on the functional and clinical outcome have been initiated. Among them is the ILUMIEN IV trial20. This large multicentre trial has been set up to recruit ~3,000 patients with complex coronary artery disease comparing clinical outcome of OCT- vs. angiography-guided PCI with a predefined OCT-guided PCI algorithm.
The DEFINE PCI study (Physiologic Assessment of Coronary Stenosis Following PCI study [NCT03084367]) uses the instantaneous wave-free ratio (iFR) to assess the functional result after conventional stenting. Within this study, blinded iFR pullbacks in patients with a satisfactory angiographic result will be used to determine how often conventional PCI misses a functionally significant lesion (iFR <0.90).
Limitations
A clear limitation is the small sample size and the non-randomised observational nature, which limits statistical power and could theoretically introduce a selection bias. Therefore, conclusions drawn from these results are hypothesis-generating and presented to highlight the issue and probable mechanisms. This optimisation strategy increased final total stent length on average by 30 mm to a total stent length of ~70 mm. Traditionally, stent length has been correlated with adverse long-term outcome, but this was of particular concern for bare metal and first-generation drug-eluting stents21,22. Improved long-term clinical outcomes have been observed in the newer-generation DES used in our study21, but further data are needed to clarify whether this increase in stent length and associated improved final FFR impact advantageously on clinical outcome. Larger randomised trials are planned and will be valuable in determining the clinical impact of any final combined imaging/physiology assessment.
Conclusions
This small consecutive series demonstrates that, despite a satisfactory angiographic result after complex PCI, up to 60% of patients had a poor functional result (FFR <0.90). The proposed FFR/OCT-guided PCI optimisation algorithm used to guide subsequent treatment was safe and demonstrated the potential to improve final FFR.
| Impact on daily practice Despite a satisfactory angiographic result, a significant number of patients undergoing complex conventional PCI may have a poor functional result. Our preliminary data suggest that the results of PCI can be optimised using an imaging/physiology-guided PCI protocol. Larger randomised trials are necessary to confirm the beneficial effects on subsequent clinical outcome but application of an FFR/OCT-guided PCI optimisation protocol could become a standard of care in complex patients. |
Funding
Partially funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre.
Conflict of interest statement
A. Banning has received unrestricted research funding from Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Methods.
Supplementary Table 1. QCA - overall population (N=35).
Supplementary Table 2. FFR before and after PCI optimisation according to performed interventions in Group 1A.
Supplementary Table 3. Incidence of vessel complications in patients with an FFR >0.90 (Group B).
Supplementary Table 4. Reasons for post-PCI optimisation and performed interventions in Group 1A.
To read the full content of this article, please download the PDF.

