
Abstract
Aims: This study aimed to investigate the diagnostic efficacy of optical coherence tomography (OCT) in identifying functional significance via fractional flow reserve (FFR) compared with that of intravascular ultrasound (IVUS).
Methods and results: We investigated 203 de novo intermediate coronary lesions of 186 patients who underwent frequency-domain OCT, IVUS and FFR measurements. Diagnostic efficacy of the minimal lumen area (MLA) obtained by OCT (OCT-MLA) and IVUS (IVUS-MLA) in predicting an FFR <0.75 was evaluated. Receiver operating characteristic curve analysis showed that OCT-MLA had significantly better diagnostic efficacy than IVUS-MLA in identifying functional ischaemia. OCT analysis revealed that the incidence of false positives (OCT-MLA ≤1.39 mm2 and FFR ≥0.75) was 46% (41/90), whereas the incidence of false negatives (OCT-MLA >1.39 mm2 and FFR <0.75) was 19% (22/113). Multivariate analysis showed that older age, non-left anterior descending artery and smaller angiographic reference diameter were independent predictors of false-positive results using the OCT-MLA criteria, whereas younger age and low left ventricular ejection fraction were independent predictors of false-negative results.
Conclusions: Intravascular imaging is not interchangeable with FFR in clinical decision making. However, OCT may have superior efficacy to IVUS in detecting functional ischaemia. Discrepancies between OCT-MLA and FFR should be taken into account for OCT-guided decision making.
Abbreviations
AUC: area under the curve
CI: confidence interval
CSA: cross-sectional area
EEM: external elastic membrane
FFR: fractional flow reserve
IVUS: intravascular ultrasound
LAD: left anterior descending artery
LVEF: left ventricular ejection fraction
MLA: minimal lumen area
OCT: optical coherence tomography
OR: odds ratio
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
ROC: receiver operating characteristic
Introduction
Fractional flow reserve (FFR) is the standard in decision making for revascularisation in catheter laboratories and has become part of the clinical guidelines for the assessment of the physiological significance of epicardial coronary stenosis1. Intravascular ultrasound (IVUS) is one of the most utilised intracoronary imaging modalities for the evaluation of anatomical and morphological plaque characteristics and guides decision making for revascularisation1-3. The relationships between physiological stenosis severity and FFR and IVUS parameters, such as minimal lumen area (MLA), minimal lumen diameter and percent stenosis area, have been thoroughly investigated4-7. However, the power of IVUS parameters to detect functional significance is not sufficient to substitute FFR values in the assessment of the physiological significance of coronary stenosis. Optical coherence tomography (OCT) provides higher image resolution than IVUS and thus enables better delineation of the luminal-intima boundary and lumen area measurements with excellent reproducibility8,9. Although several small studies have investigated the predictive power of OCT-derived parameters, especially MLA, compared with that of FFR values10-14, few studies have performed a head-to-head comparison of the diagnostic efficacy of IVUS and OCT parameters on an identical lesion set. Therefore, in this study, we sought to achieve the following: 1) perform a head-to-head comparison of OCT with IVUS in the prediction of the haemodynamic significance of coronary artery stenosis; and 2) identify the factors that affect the relationship between the FFR value and the OCT parameters. Additionally, considering the higher resolution of OCT and its predicted superiority in discriminating small lumen areas, we hypothesised that OCT would exhibit better diagnostic efficacy than IVUS in lesions with more severe stenosis (FFR <0.75) than in those with an FFR ≤0.80.
Methods
STUDY POPULATION
The institutional database of cardiac catheterisations performed at Tsuchiura Kyodo General Hospital between September 2011 and August 2016 was screened to identify patients with coronary stenosis for whom frequency-domain OCT imaging, IVUS imaging and FFR measurements were performed during the same procedure in either diagnostic or therapeutic catheterisation. Patients were eligible for the analysis if they fulfilled the following criteria: >20 years old; physiological assessment by pressure wire for de novo coronary lesions indicating intermediate stenosis, which was defined as a visual estimation of 30% to 80% angiographic diameter stenosis; and written consent for both OCT and IVUS imaging for stenotic lesions. The culprit lesions of acute coronary syndrome were not included. In addition, lesions in extremely tortuous vessels or with heavy calcification were excluded because of expected difficulty in advancing the OCT or IVUS catheters. We also excluded patients with angiographically significant left main disease, a history of coronary artery bypass surgery, renal insufficiency with a baseline serum creatinine level >1.5 mg/dL or congestive heart failure. Lesions requiring balloon angioplasty prior to intracoronary imaging, tandem lesions, and lesions that the imaging catheter failed to cross or lesions with unsatisfactory OCT/IVUS image quality were also excluded. Thus, a total of 203 coronary lesions from 186 patients were eligible for the final analysis (Figure 1). Baseline patient characteristics were collected by reviewing medical charts. The study protocol was approved by the institutional review board, and all patients provided written informed consent prior to catheterisation.
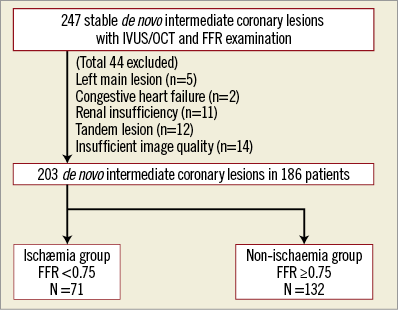
Figure 1. Patient population. A total of 247 intermediate coronary lesions in 225 patients undergoing IVUS/OCT and FFR examination were selected from the institutional database. After applying the exclusion criteria, 203 de novo intermediate coronary lesions were included in the analysis. The lesions were divided into two groups based on the FFR cut-off value, the ischaemia group (FFR <0.75, n=71) and the non-ischaemia group (FFR ≥0.75, n=132).
CARDIAC CATHETERISATION
Each patient initially underwent standard selective coronary angiography via the radial artery for the assessment of coronary anatomy using a 6 Fr system. Coronary angiograms were quantitatively analysed using a CMS-MEDIS quantitative coronary angiography (QCA) system (Medis medical imaging systems, Leiden, the Netherlands) to measure the lesion length, minimum lumen diameter, reference lumen diameter and percent diameter stenosis at the target lesion. All patients received a bolus injection of heparin (5,000 IU) before the procedure, and an additional bolus injection of 2,000 IU was administered every hour as needed to maintain an activated clotting time of >250 seconds. An intracoronary bolus injection of nitroglycerine (0.2 mg) was administered at the start of the procedure and repeated every 30 minutes. QCA measurements were performed in diastolic frames from orthogonal projections. The OCT/IVUS imaging and FFR measurements were performed prior to percutaneous coronary intervention (PCI) for the target lesion and after the PCI procedure for non-target vessels in patients treated by PCI or during diagnostic coronary angiography in patients in whom PCI was being deferred.
FFR MEASUREMENTS
For FFR measurements, a RadiAnalyzer Xpress console with PressureWire™ Certus™ (St. Jude Medical, St. Paul, MN, USA) was employed to measure the distal coronary pressure. FFR was measured in vessels that were clinically indicated for evaluation, presenting between 30% and 80% angiographic stenosis on visual estimation. After the administration of nitroglycerine, a pressure-monitoring guidewire was advanced distal to the stenosis. Hyperaemia was induced by intravenous infusion of adenosine 5′-triphosphate at a rate of 160 μg·kg-1·min-1. FFR was calculated by dividing the mean distal pressure by the mean aortic pressure during stable maximal hyperaemia.
OCT IMAGE ACQUISITION AND ANALYSIS
OCT images were acquired using frequency-domain OCT systems (ILUMIEN™ [St. Jude Medical] or Lunawave® [Terumo, Tokyo, Japan]). The technique of OCT image acquisition has been described elsewhere8,9. The OCT imaging data were digitally stored and analysed offline. All OCT images were analysed using proprietary software (LightLab Imaging and Terumo) based on expert consensus documents at the Tsuchiura Kyodo Hospital OCT Laboratory8,9. The lumen contour was semi-automatically traced with proprietary software on cross-sectional images, and the contour was manually corrected by the investigator if needed. The cross-section with the smallest luminal area value was defined as the MLA. Qualitative parameters were assessed by two experienced institutional OCT laboratory technicians who were blinded to the angiographic data and clinical characteristics.
IVUS IMAGE ACQUISITION AND ANALYSIS
IVUS imaging was performed for all lesions prior to any interventional procedure using a 40-MHz IVUS catheter (Atlantis SR Pro2™ or OptiCross™; Boston Scientific, Marlborough, MA, USA). The catheter was advanced distal (>10 mm) to the target lesion up to a distal landmark documented by angiography. Automated pullback at 0.5 mm/s was then performed from this point to the aorto-ostial junction. IVUS images were digitally stored for subsequent offline analysis. Quantitative IVUS analysis was performed according to the American College of Cardiology Clinical Expert Consensus Document3. A discrete interface at the border between the media and the adventitia corresponds closely to the location of the external elastic membrane (EEM). Using planimetry software (QCU-CMS; Medis medical imaging systems), the cross-sectional areas (CSAs) were measured. Lumen measurements were performed semi-automatically using the interface between the lumen and the leading edge of the intima. In a similar way to that previously described for OCT, the contour was manually corrected by the investigator if needed. IVUS-MLA was defined as the minimum lumen CSA. Plaque plus media CSA was calculated as the EEM-CSA minus the lumen CSA, and plaque burden was calculated as the plaque plus media divided by the EEM-CSA. Proximal and distal references were single slices with the largest lumen and smallest plaque burden within 10 mm proximally and distally, respectively, but before any large side branch. Two experienced investigators (E. Usui and T. Yonetsu) blinded to the angiographic, OCT and other clinical data analysed the IVUS images. In both OCT and IVUS analysis, the discordance between the investigators was resolved by consensus reading or the mean value was calculated.
STATISTICAL ANALYSIS
All statistical analyses were performed using SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA) and R statistics version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria). Patient demographics are presented as n (%) where appropriate. Categorical data are expressed as absolute frequencies and percentages and were compared using the χ2 or Fisher’s exact test, as appropriate. Continuous variables are expressed as the mean±standard deviation for normally distributed variables or as the median (25th-75th percentile) for non-normally distributed variables and were compared using the Student’s t-test or the Mann-Whitney U test, respectively. Correlations between the intracoronary anatomical parameters and FFR were assessed by Pearson correlation analysis. Receiver operating characteristic (ROC) curve analyses were performed to identify the optimal cut-off values of angiographic, IVUS and OCT parameters for the prediction of haemodynamic significance with maximum accuracy. Sensitivity, specificity, positive predictive values and negative predictive values were determined with 95% confidence intervals. We further evaluated the predictors of discrepancy for OCT-MLA and FFR using univariate and multivariate logistic regression analyses (stepwise forward method). The variables found to be associated in the univariate analysis (p<0.20) were included in the multivariate model. A generalised estimating equations approach was used to consider within-subject correlations resulting from the analysis of multiple vessels within a single patient. P<0.05 indicated statistical significance.
Results
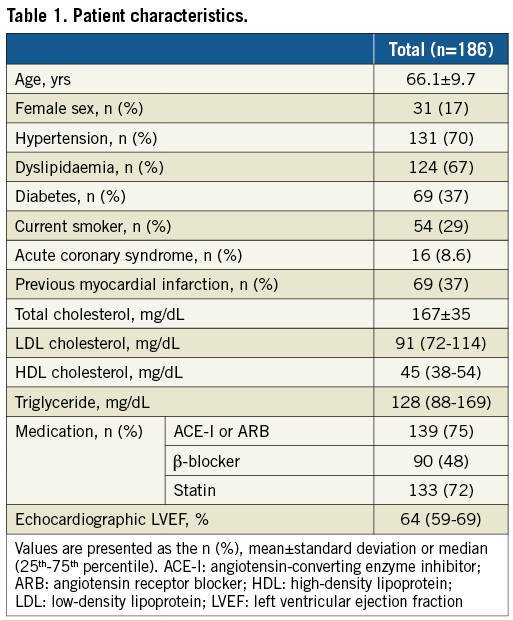
The baseline characteristics of the study population are summarised in Table 1. For a total of 203 lesions, the mean FFR value, median FFR value and mean % diameter stenosis were 0.76±0.10, 0.78 (IQR 0.72-0.83) and 54.8±8.79%, respectively. Among the 203 lesions, the FFR was <0.75 in 71 lesions (35%, ischaemia group) and ≥0.75 in 132 lesions (65%, non-ischaemia group). The FFR was ≤0.80 in 140 lesions (69%) and >0.80 in 63 lesions (31%).
DIFFERENCES IN ANGIOGRAPHIC, OCT AND IVUS FINDINGS BASED ON FFR SEVERITY
The angiographic, OCT and IVUS findings for all 203 lesions are exhibited in Table 2. Lesions in the left anterior descending artery (LAD) were more prevalent in the ischaemia group than in the non-ischaemia group. The results of the QCA analysis indicated that the minimum lumen diameter in the ischaemia group was significantly smaller than that in the non-ischaemia group. Additionally, the % diameter stenosis in the ischaemia group was significantly greater than that in the non-ischaemia group, whereas a modest trend towards a smaller reference diameter and longer lesion length was observed in the ischaemia group. OCT analysis showed that the ischaemia group exhibited a smaller MLA, greater percent stenosis area and greater maximum lipid arc than the non-ischaemia group. IVUS analysis showed that the ischaemia group exhibited a smaller MLA and greater percent plaque burden than the non-ischaemia group.
EFFICACY OF EACH ANATOMICAL PARAMETER IN PREDICTING FUNCTIONAL SIGNIFICANCE
ROC curve analysis revealed that the best cut-off values of angiographic % diameter stenosis, IVUS-MLA and OCT-MLA for the prediction of the presence of ischaemia (FFR <0.75) were 57.8% (area under the curve [AUC]: 0.609, 95% confidence interval [CI]: 0.527-0.691), 2.57 mm2 (AUC: 0.615, 95% CI: 0.534-0.696), and 1.39 mm2 (AUC: 0.732, 95% CI: 0.660-0.804), respectively (Figure 2A). The AUC of OCT-MLA for the prediction of ischaemia was significantly greater than that of IVUS-MLA (p<0.01) (Figure 2A). For the prediction of FFR ≤0.80, the AUC of OCT-MLA was not significantly greater than that of IVUS-MLA (p=0.13) (Figure 2B).
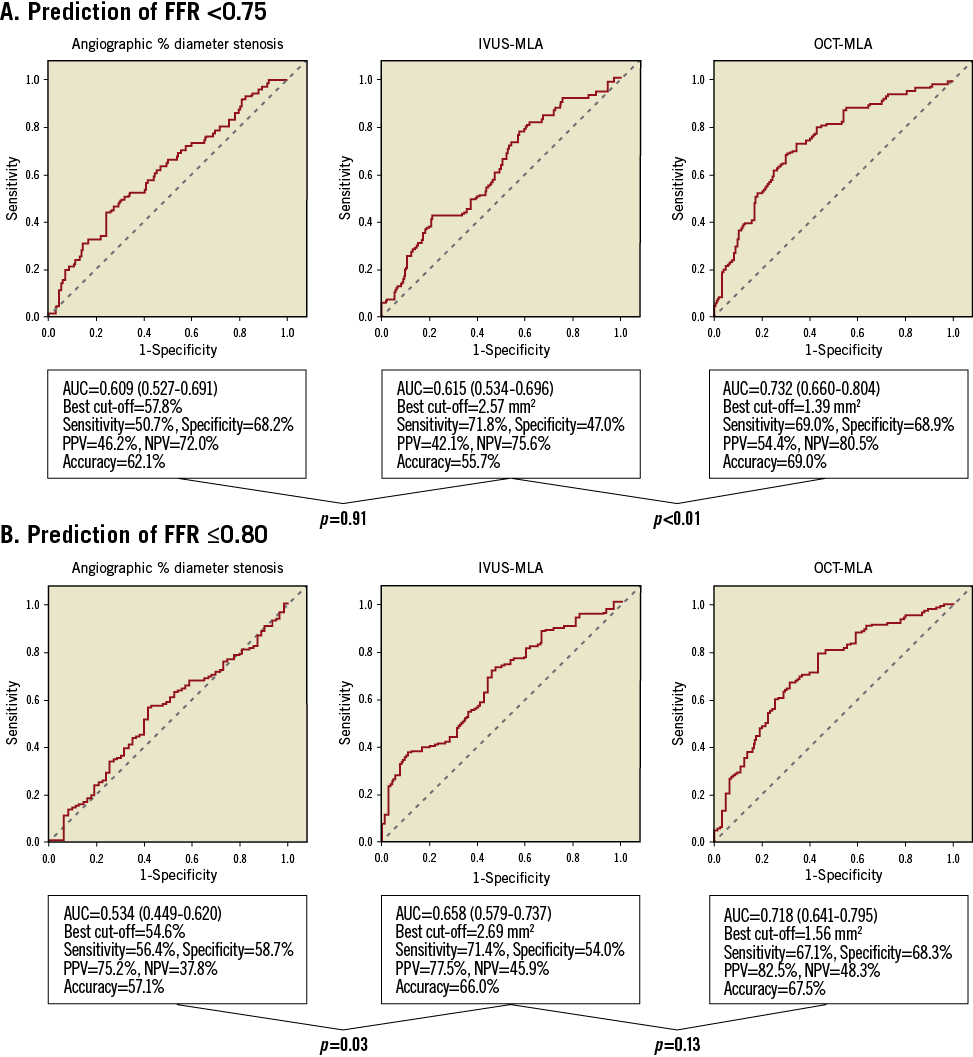
Figure 2. ROC curves of anatomical parameters to predict (A) FFR <0.75, and (B) FFR ≤0.80. The left panels show the ROC curves for angiographic % diameter stenosis. The central panels show the ROC curves for IVUS-derived MLA. The right panels show the ROC curves for OCT-derived MLA.
DETERMINANTS OF THE DISCREPANCY IN DECISION MAKING BY OCT-MLA AND FFR
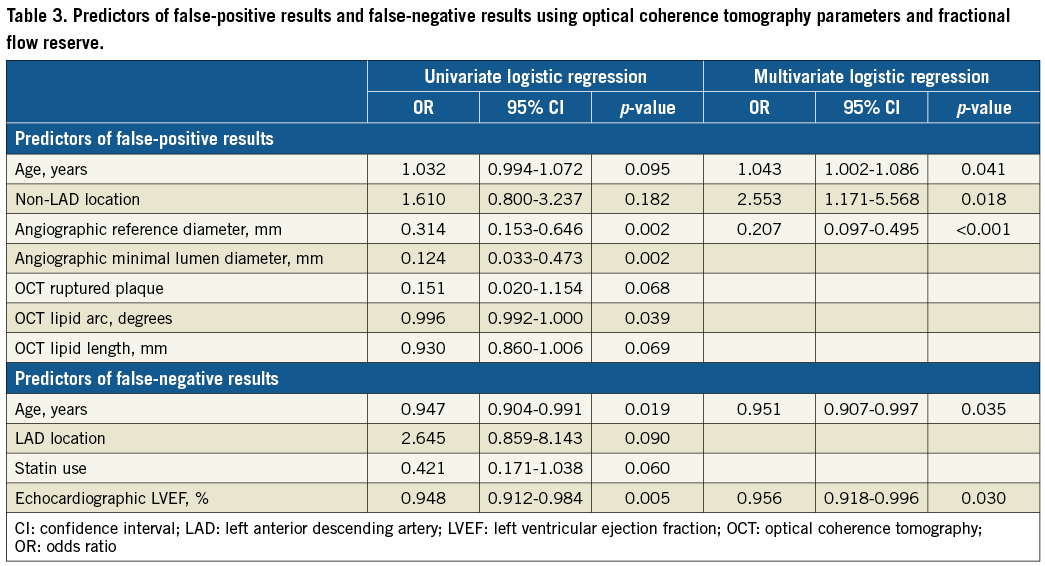
There was a weak but positive correlation between IVUS-MLA and FFR (R=0.259, p<0.001), whereas the correlation was more apparent between OCT-MLA and FFR (R=0.465, p<0.001) (Figure 3). IVUS analysis showed that the incidence of false positives (IVUS-MLA ≤2.57 mm2 and FFR ≥0.75) was 58% (70/121) and the incidence of false negatives (IVUS-MLA >2.57 mm2 and FFR <0.75) was 24% (20/82) (Figure 3A). OCT analysis showed that the incidence of false positives (OCT-MLA ≤1.39 mm2 and FFR ≥0.75) was 46% (41/90) and the incidence of false negatives (OCT-MLA >1.39 mm2 and FFR <0.75) was 19% (22/113) (Figure 3B). OCT showed a decreasing trend for false-positive results compared with IVUS (p=0.077). Multivariate analysis showed that age (odds ratio [OR]: 1.043; 95% CI: 1.002 to 1.086; p=0.041), non-LAD (OR: 2.553; 95% CI: 1.171 to 5.568; p=0.018), and angiographic reference diameter (OR: 0.207; 95% CI: 0.097 to 0.495; p<0.001) were independent predictors of false-positive results, whereas age (OR: 0.951; 95% CI: 0.907 to 0.997; p=0.035), and left ventricular ejection fraction (LVEF) (OR: 0.956; 95% CI: 0.918 to 0.996; p=0.030) were independent predictors of false-negative results using OCT-FFR (Table 3).
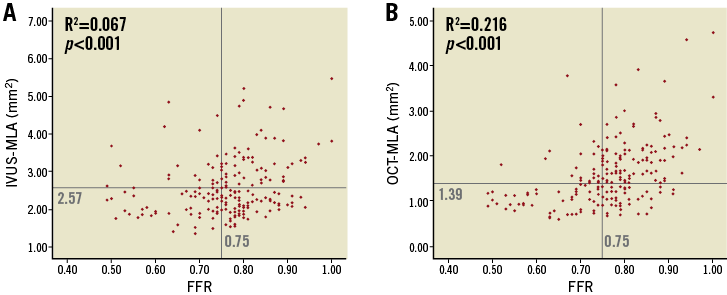
Figure 3. Relationship between IVUS-MLA and FFR (A) as well as OCT-MLA and FFR (B).
Discussion
The main findings of this study were as follows: 1) OCT-MLA showed significantly better diagnostic efficacy than IVUS-MLA in identifying functional ischaemia, defined as FFR <0.75, while the diagnostic efficacy between OCT-MLA and IVUS-MLA was not significantly different when the functional ischaemia was defined as FFR ≤0.80; 2) anatomical and physiological mismatch was not uncommon based on either the IVUS or OCT criteria; and 3) the predictors of false-positive OCT-derived decisions were older age, non-LAD and smaller reference diameter, whereas the predictors of false-negative OCT-derived decisions were younger age and lower LVEF.
COMPARISON BETWEEN OCT-MLA AND IVUS-MLA FOR THE PREDICTION OF PHYSIOLOGICAL ISCHAEMIA
The current guideline recommendations2 recognise IVUS as an accurate method for optimising stent implantation. However, its efficacy in predicting functional severity of non-left main coronary lesions remains elusive. Although several studies, including large international multicentre trials, have investigated the relationship between FFR severity and IVUS parameters4-7, IVUS parameters are not interchangeable with FFR-guided decision making for revascularisation.
There are still few data regarding the diagnostic efficacy of IVUS versus OCT in detecting functional stenosis severity in an identical lesion set. Our head-to head investigation focused on the diagnostic capability of OCT and IVUS for functional stenosis and showed that OCT-MLA was significantly better than IVUS-MLA for identifying ischaemia when the ischaemic threshold was defined as FFR <0.75. However, no significant difference was detected between the two methods in identifying ischaemia with a threshold of FFR ≤0.80. The AUC of OCT-MLA was only 0.732, and the diagnostic accuracy was 69%, suggesting its limited utility for the prediction of ischaemia; thus, the OCT-MLA criteria are unlikely to substitute FFR in guiding decision making for revascularisation. However, in daily clinical practice, penetration of physiological assessment with a pressure wire remains low, potentially due to contraindication for adenosine infusion, haemodynamic complications, or economic reasons such as incomplete reimbursement. In such cases, predicting physiological ischaemia by using imaging modalities may be beneficial. The difference in diagnostic efficacy between IVUS and OCT based on FFR threshold values might be explained as follows. 1) Our study population included more functionally significant lesions than the previous studies, thereby affecting the positive and the negative predictive values. This may also explain why our suggested OCT-MLA cut-off values for the prediction of ischaemia were smaller than those of previous studies10-14. 2) High-resolution OCT images might help us to identify or quantify tight stenosis accurately, since measurement error may influence the decisions involving lesions with small lumen areas compared with those with relatively large lumen areas. The better efficacy of OCT in discriminating the lumen-vessel boundary concurs with the results from a previous small-sized study that performed a head-to-head comparison of IVUS-MLA with OCT-MLA for predicting ischaemia11.
DETERMINANTS OF THE DISCREPANCY OF OCT-MLA AND FFR
The current study suggested that older age correlates with false-positive results and that younger age is associated with false-negative results. Age-related changes in cardiac structure, including the progression of interstitial fibrosis and coronary disease, such as endothelial or microvascular dysfunction, may influence FFR15. Non-LAD lesions and smaller angiographic reference vessel diameters were independent predicters of false-positive results. Our results are in line with those reported by previous studies4,6,16. Lower LVEF was an independent predictor of false-negative results. It has been reported that, theoretically, FFR shows an inverse relation with LVEF because of its positive relation with left ventricular end-diastolic pressure17. On the other hand, Kobayashi et al reported that reduced LVEF has no influence on the FFR value unless the stenosis is very tight18. Their explanation of this phenomenon is that less severe stenosis with reduced LVEF may be just as likely to occur in vessels subtending viable myocardium as in patients with preserved EF. Further studies including the FFR data in relation to left ventricular wall motion locality and end-diastolic pressure are needed to clarify this issue. Although not statistically significant (p=0.077), OCT showed a strong decreasing trend for the false-positive detection of lesions with an FFR <0.75 compared with IVUS. This finding may also indicate the superiority of OCT to IVUS for decision making for revascularisation and might contribute to the reduction of unnecessary revascularisation.
Study limitations
The results of the present study should be interpreted with consideration of certain limitations. First, this study was a single-centre, retrospective study enrolling only a Japanese population. Second, the decision to perform FFR measurements was at the discretion of the operator. Third, we selected lesions that were evaluated with both IVUS and OCT during the same procedure, which may have resulted in selection bias. Fourth, we compared IVUS and OCT by exclusively using MLA in this study. Other lesion-related factors such as plaque length or morphology may be associated with functional significance when the analysis is performed in a larger study population. Fifth, the present study used an FFR cut-off value of 0.75. Although the value is a specific threshold for inducible and reversible ischaemia based on the DEFER study19, an FFR ≤0.80 is the threshold widely used for the indication of revascularisation in large clinical trials20,21. Thus, additional large-scale studies including adequate numbers of functionally insignificant lesions are required to confirm our results and to establish better predictive models.
Conclusions
Intravascular imaging is not interchangeable with FFR in clinical decision making for revascularisation; however, OCT may have superior efficacy to that of IVUS in detecting functionally significant stenosis, particularly when detecting severe stenosis, and may reduce the incidence of unnecessary coronary intervention. Factors leading to the discrepancy between the OCT-MLA criteria and FFR should be taken into account when OCT quantification is used to guide decision making for revascularisation.
| Impact on daily practice OCT can provide higher image resolution than IVUS. The present study showed that OCT may have superior efficacy to IVUS in detecting functional ischaemia although intravascular imaging is not interchangeable with FFR in clinical decision making. Discrepancies between OCT-MLA and FFR should be taken into account for OCT-guided decision making; the predictors of false-positive OCT-derived decisions were older age, non-LAD and smaller reference diameter, whereas the predictors of false-negative OCT-derived decisions were younger age and lower LVEF. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

