Abstract
Aims: Fractional flow reserve (FFR) of <0.8 or 0.75 is currently used to guide revascularisation in lesions with intermediate coronary stenosis. We assessed whether there is an intravascular ultrasound (IVUS) measurement that can reliably be used to predict when patients should undergo intervention.
Methods and results: The analysis included 92 intermediate lesions (84 patients) located in vessel diameters >2.5mm. Positive FFR was considered present at <0.8 and 0.75. IVUS minimum lumen area (MLA) was correlated to the FFR findings in intermediate lesions with 40-70% stenosis. The mean FFR value was 0.89±0.08. Twenty-four patients (26.1%) had FFR <0.8; 17 (18.5%) <0.75. Positive correlations between FFR and IVUS measurements included MLA (r = 0.34, p<0.001), minimum lumen diameter (MLD) (r=0.31, p=0.004), lesion length (r=–0.5, p<0.001), and area stenosis (r=–0.31, p=0.01). There was no significant correlation between FFR and quantitative coronary angiography in MLD (r=0.19, p=0.06), diameter stenosis (r=0.08, p=0.4), or lesion length (r=–0.14, p=0.17). A receiver operating characteristic curve identified MLA <2.8 mm2 (sensitivity 79.7%, specificity 80.3%) as the best threshold value for FFR <0.75; and MLA <3.2mm2 as best for FFR <0.8 (sensitivity 69.2%, specificity 68.3%).
Conclusions: Anatomic measurements of intermediate coronary lesions obtained by IVUS show a moderate correlation to FFR values, although they differ according to vessel size. IVUS MLA may be used as an alternative to FFR when assessing the need for intervention in intermediate coronary lesion. Vessel size, however, should always be taken into account.
Introduction
An intermediate coronary stenosis, defined as a luminal narrowing with a diameter stenosis of 40-70% on angiography, is a source of controversy with regard to the appropriate criteria for undertaking revascularisation. Measurement of the degree of stenosis with visual estimation from the angiogram or by quantitative coronary angiography (QCA) is inaccurate; therefore intravascular ultrasound (IVUS) and functional assessment of coronary stenosis by fractional flow reserve (FFR) are currently used to define the severity of such lesions. Clinical decision based on FFR of intermediate coronary stenosis is safe and results in an excellent clinical outcome1,2. Similarly, deferring intermediate native coronary artery lesion intervention based on IVUS guidance with minimum lumen cross sectional area (MLA) >4mm2 correlates with a low event rate3. To date, few data are available regarding the relationship between anatomical IVUS parameters and functional FFR results. This study was undertaken to evaluate the relationship between QCA analysis, IVUS parameters, and FFR values in patients with intermediate coronary stenosis. We also sought to determine the IVUS anatomical criteria and threshold values associated with both FFR <0.8 as recently proposed2 and FFR <0.75 as previously validated1.
Methods
From July 2007 to October 2009, 92 intermediate lesions in major epicardial coronary vessels of 84 patients were studied consecutively by QCA, IVUS, and FFR during diagnostic coronary angiography. Intermediate coronary stenosis was defined as a stenosis of 40-70% by QCA. Patients with acute myocardial infarction, left main lesions, saphenous vein graft lesions, lesions in vessels <2.5mm in diameter, or >1 lesion in the vessel studied were excluded. Written informed consent for all procedures was obtained from each patient. Clinical data regarding cardiac risk factors, left ventricular function, and prior non invasive studies were also collected.
QCA analysis was performed by an independent technologist blinded to the results of both IVUS and FFR. A computer-assisted, automatic contour detection technique (CAAS Quantitative Coronary Angiography for Research, Pie Medical Imaging BV, Maastricht, The Netherlands) was employed. The outer diameter of the contrast-filled catheter served as the calibration standard. After selection of the optimal projection displaying the most severe stenosis, the percent diameter stenosis at end diastole, minimum lumen diameter (MLD), reference vessel diameter, and lesion length were measured. Lesion length was calculated as the distance between the proximal and distal shoulder in the projection demonstrating the stenosis with the least foreshortening.
IVUS studies were performed using one of the two commercially available systems. The Boston Scientific (Minneapolis, MN, USA) system incorporated a 40-MHz single element bevelled transducer (Atlantis SR) rotating at 1,800 rpm coupled with Clear View (CVIS, Sunnyvale, CA, USA) or Galaxy (Boston Scientific) consoles. The Volcano Therapeutics system (Rancho Cordova, CA, USA) incorporated a phased array immobile set of crystals arranged circularly around the catheter and was activated sequentially at 20 MHz. All IVUS images were recorded after administration of intracoronary nitroglycerin 200µg. The transducer was pulled back from the distal coronary artery through the target stenosis and to the proximal portion at 0.5 or 1.0mm/s. Images were recorded on videotapes or compact discs for offline analysis. Quantitative analysis of the IVUS images was performed by a skilled interpreter using computerised planimetry with TapeMeasure 4.2.16C (INDEC Systems, Inc., Mountain View, CA, USA). Lumen cross sections were measured at the most stenotic site with the smallest lumen. The reference vessel cross sectional area was measured by tracing the leading edge of the adventitia with the most visually normal cross section (largest lumen with the least plaque) within 10-mm proximal and distal to the lesion. A distal reference was used for ostial lesions. The area stenosis was calculated as reference lumen area minus MLA, divided by reference lumen area.
To assess FFR, a 0.014-inch pressure guidewire (Radi Medical System, Upsala, Sweden) was employed. Distal pressure was measured immediately distal to the stenosis during a period of maximum hyperaemia induced by intravenous adenosine (140µg/kg/min for the right coronary artery and 180µg/kg/min for the left coronary artery). Aortic pressure was measured through the guiding catheter (6 or 7Fr). FFR was calculated as the ratio of the coronary pressure distal to the lesion measured by the pressure wire to the mean aortic pressure measured by the guiding catheter. Based on results of these three methods, we correlated the IVUS and QCA parameters with FFR results. The MLA by IVUS that was most closely associated with 2FFR ratios: <0.8 and <0.75 was sought. The decision for treatment of any lesion was made by the operator.
Statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, NC, USA). Continuous variables are expressed as mean ±standard deviation and categorical variables as frequency and percentages. Differences between continuous variables were assessed with Student’s t-test. Categorical variables were compared with the chi-square test or Fisher’s exact test when appropriate. Significance was set at p <0.05. The relationship and variability between FFR and the IVUS or QCA parameters were analysed by Pearson correlation analysis to define correlation coefficients between FFR and IVUS or QCA index of lesion severity. Logistic regression and receiver operating characteristic curve analysis were performed to establish the value of IVUS indices that were most predictive of FFR <0.8 or <0.75.
Results
Ninety-two intermediate coronary lesions in 84 patients were analysed. The baseline clinical characteristics and lesion-specific characteristics are presented in Table1. The mean age was 63.9±11.8 years, and 49 patients (58.3%) were male. Sixty-one lesions (66.3%) were located in the left anterior descending artery territory. The mean FFR value was 0.89±0.08. In 24 stenoses (26.1%), the FFR was <0.8 and in 17 (18.5%) the FFR was <0.75. The mean MLA was 3.6±1.1mm2, and the mean diameter stenosis by QCA was 47.5±9.8%. The MLD by IVUS was 1.8±0.35 mm, and by QCA was 1.7±0.48 mm.
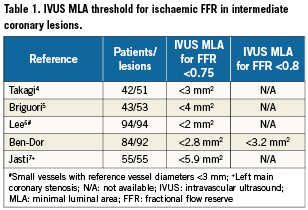
The relationship of MLA by IVUS and FFR is graphically displayed in a scatter plot (Figure 1). Importantly, MLA >4mm2 was nearly always associated with FFR >0.8. On the other hand, 42 lesions (67.7%) with MLA <4 mm2 had FFR that exceeded 0.8 and 47 (75.8%) had FFR that exceeded 0.75. Eleven lesions (37.9%) with MLA <3mm2 had FFR that exceeded 0.8, while and 17 (58.6%) had FFR that exceeded 0.75. The relationship of FFR and IVUS measurements as compared by linear regression is detailed in Figures 2A-D, and demonstrates a significant, moderately association. There was no significant correlation between FFR and MLA in lesions with vessel reference diameters of 2.5-3mm (r=0.11, p=0.5). There was a significant correlation between FFR and MLA in lesions with vessel reference diameters of 3-3.5 mm and >3.5mm (r=0.5, p<0.001, r=0.4, p=0.01, respectively) (Figure 3). No such relationship, however, was demonstrated when FFR and QCA were compared (Figure 4). The probability of an ischaemic FFR <0.75 or <0.8 at various sizes of MLA by IVUS is depicted in Figure 5. For a stenosis with MLA >4mm2, the likelihood of FFR <0.75 was vanishingly small, but below that dimension the likelihood of FFR <0.75 increased with decreasing MLA (Figure 4).
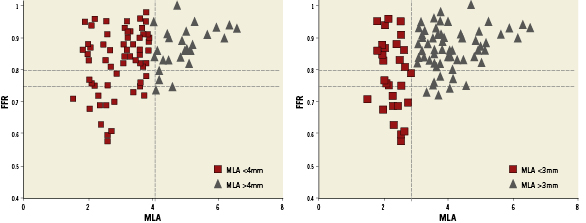
Figure 1. Plots presenting the relationship between fractional flow reserve (FFR) and minimum lumen area (MLA).
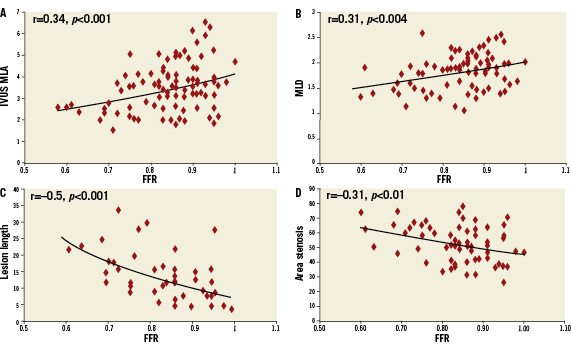
Figure 2. Plots of relationship between FFR and intravascular ultrasound (IVUS) parameters: A) MLA; B) Minimum luminal diameter (MLD); C) Lesion length; D) Area stenosis.
Figure 3. Plots of relationship between FFR and MLA by reference vessel diameter.
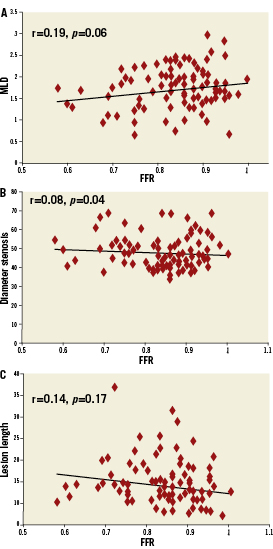
Figure 4. Plots of relationship between FFR and quantitative coronary angiography (QCA) parameters: A) MLD; B) Diameter stenosis; C) Lesion length.
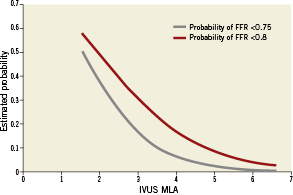
Figure 5. The probability of ischaemic FFR <0.75 or <0.8 at various sizes of MLA by IVUS.
Receiver operating characteristic curve analysis was conducted to identify MLA by IVUS with the best discriminatory value for identifying FFR <0.75 and FFR <0.8 (Figure 6). MLA <2.82mm2 (sensitivity 79.7%, specificity 80.3%) (AUC=0.76) was the best threshold value for identifying FFR <0.75. Similarly, MLA <3.2mm2 (sensitivity 69.2%, specificity 68.3%) (AUC=0.74) was the best discriminator for FFR <0.8. Receiver operating characteristic curve analysis identified MLA <2.54mm2 (AUC=0.68) as the best threshold value for FFR <0.75 in lesions with reference vessel diameters of 2.5-3mm, MLA <2.88mm2 (AUC=0.91) in lesions with reference vessel diameters of 3-3.5 mm, and MLA <3.7mm2 (AUC=0.83) in lesions with reference vessel diameters >3.5 mm (Figure 7).
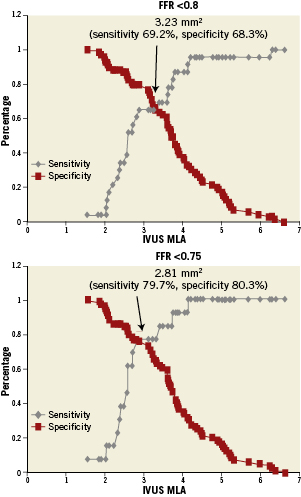
Figure 6. Representation of sensitivity and specificity and functional cut-off values over the spectrum of MLA by IVUS and ischaemic FFR <0.75 and FFR <0.8.
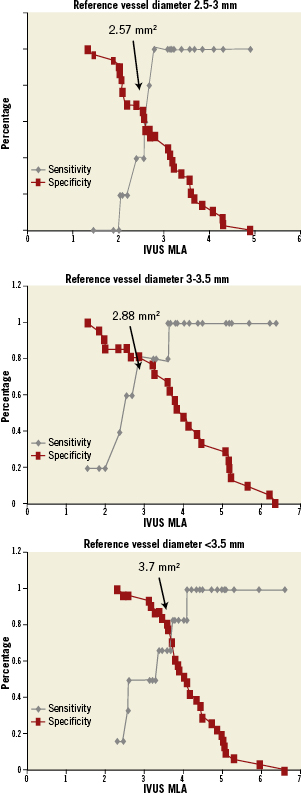
Figure 7. Representation of sensitivity and specificity and functional cut off values over the spectrum of MLA by IVUS and ischaemic FFR <0.75 in different reference diameters.
Of the 24 intermediate stenoses (26%) in this trial with FFR <0.8, 4 had MLA >4 mm2. All but one was treated with percutaneous coronary intervention. In that case FFR was 0.79 and MLA was >4mm2. Of the 68 stenoses with FFR >0.80, 37 (54.4%) had MLA <4mm2 and 11 (16.1%) underwent a revascularisation procedure; the mean MLA of those 11 patients was 2.7±0.9mm2. In addition, six had a positive non-invasive test for ischaemia (Figure 8).
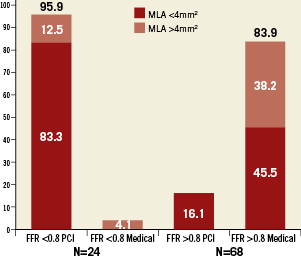
Figure 8. Treatment of lesions with FFR <0.8 vs. FFR >0.8.
Discussion
Our data support MLA measured by IVUS as an acceptable surrogate for measuring the severity and potential haemodynamic importance of an intermediate stenosis detected by FFR. Importantly, if the MLA exceeds 4 mm2 (the accepted IVUS criterion for a significant stenosis), FFR <0.75 or 0.80 (the accepted criteria for significant stenosis) is virtually never present. On the other hand, as the lesion narrows beyond 4 mm2, FFR declines sharply, but a substantial portion of lesions with MLA <4 mm2 are not ischaemia-producing as estimated by FFR. These results raise doubts about the necessity of treating every lesion with MLA <4 mm2.
A moderately linear correlation exists between FFR and MLA (r=0.34, p<0.001). This correlation was better in large vessels (>3mm) than in medium vessels (>2.5mm). On receiver operating character curve analysis, MLA of 2.82mm2 best correlated with FFR <0.75 and MLA of 3.2mm2 with FFR <0.8. The MLA threshold value was not an absolute number because as the vessel diameter decreased the MLA threshold decreased. We also found a significant linear correlation between FFR and IVUS lesion length (r=-0.5, p<0.001). Since longer stenoses result in a greater degree of flow obstruction, it is quite plausible that lesion length by IVUS correlates well with FFR.
Other studies have reported the correlation between IVUS and FFR. (Table 1) Takagi et al4 found results consistent with ours. They reported, in 42 patients, that the IVUS thresholds which best correlate with FFR <0.75 were MLA <3.0mm2 (sensitivity, 83.0%; specificity, 92.3%) and area stenosis >0.6 (sensitivity, 92.0%; specificity, 88.5%). Their results, however, may not directly bear on stenoses of intermediate severity on angiography, as only half of the lesions analysed were in this category. Birgouri et al5 also studied 53 intermediate lesions. They found that MLA ≤4mm2 correlated most closely with FFR <0.75 (sensitivity 92%, specificity 56%). The specificity of only 56% suggests that, as in our analysis, many patients with MLA <4mm2 did not meet FFR criteria. Taken together, the studies suggest that MLA by IVUS of <3mm2 best predicts FFR of 0.8 or 0.75. In small vessels defined as those with reference diameters <3mm we found that IVUS MLA <2.54mm2 predicts ischaemic FFR <0.75. Lee et al6 reported that IVUS MLA <2.0mm2 predicts ischaemic FFR <0.75. The difference could result from the inclusion of vessels <2.5mm and diameter stenosis >70%, which were exclusion criteria in our study. For intermediate left main stenosis, IVUS MLA <5.9mm2 had the highest sensitivity and specificity for FFR <0.75.7
A study that evaluated the correlation of IVUS and coronary flow reserve reported that a MLA ≥4.0mm2 has a high diagnostic accuracy in predicting a non-ischaemic coronary flow reserve ≥2.0.8 Studies comparing FFR to noninvasive testing suggest that ischaemic findings appear at FFR 0.72 to 0.759. Wijns et al10 studied 45 patients with angiographically ambiguous stenoses, and reported that FFR had much greater accuracy in distinguishing haemodynamically significant stenoses than exercise electrocardiogram, myocardial perfusion scintigraphy and stress echocardiography. Tests were considered positive only if they were positive before revascularisation and subsequently negative following revascularisation. They also stated that FFR <0.75 was always associated with inducible ischaemia (specificity 100%), and that FFR >0.75 excluded ischaemia related to that stenosis with few exceptions (sensitivity 90%).11-14 Taken together with the data in this report, utilising an IVUS measurement <4 mm2 is suggested. This dimension corresponds to FFR <0.75 based on our analysis, which would be MLA of 2.82mm2.
Several studies have found that deferring intervention in stenoses with FFR >0.75 is not associated with a higher rate of adverse cardiac events.15-17 Moreover, Pijls et al1 examined the long-term results of deferring intervention based on FFR ≥0.75 in 325 patients scheduled for percutaneous coronary intervention of an intermediate stenosis. They found an excellent 5-year outcome. The risk of cardiac death or myocardial infarction related to such stenoses was <1% per year and, importantly, was not decreased by stenting.
The Fractional Flow Reserve versus Angiography for Guiding PCI in Patients with Multivessel Coronary Artery Disease (FAME) investigators2 randomised 1,005 patients with multivessel coronary disease and lesions appropriate for percutaneous coronary intervention to either 1) routine measurement of FFR and stenting limited to stenoses with FFR ≤0.8 or 2) stenting of all of the lesions deemed appropriate based on the angiography. The rate of the composite endpoint of death, myocardial infarction, and repeat revascularisation at one year was significantly better for those with FFR-guided percutaneous coronary intervention. Importantly, rather than using the FFR 0.75 threshold, the investigators chose a less restrictive one (<0.8) in order to limit the number of potentially ischaemic lesions left untreated. Long-term clinical outcome of patients with a left main coronary stenosis in whom surgery was deferred on the basis of FFR values >0.80 is favourable and similar to that of patients in whom coronary artery bypass graft surgery was performed on the basis of FFR values <0.80.18 The data identifying MLA <4 mm2 as a threshold for potential ischaemia is less plentiful. Nishioka et al19 found that this dimension identified ischaemia-provoking lesions based on single photon emission computed-tomography (sensitivity 88%, specificity 90%).
A randomised trial evaluating whether to pursue intervention when the MLA is <4 mm2 by IVUS is appropriate when using IVUS MLA for decision-making in intermediate coronary stenosis. Two retrospective analyses provide some insight. Abizaid et al3 described 300 patients with intermediate lesions in whom intervention was deferred based on an IVUS MLA >4 mm2 showing an overall event rate at 1-year follow-up of 8%. Furthermore, IVUS MLA was an independent predictor for an adverse event (death, myocardial infarction, or target lesion revascularisation). The event rate was only 4.4% in patients with MLA >4 mm2. Recently the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) trial20 followed for three years 700 patients with acute coronary syndrome. The major cardiovascular event rate was quite low at 11.6%, and one correlation of non-culprit lesion related events was MLA <4 mm2. The evidence that percutaneous coronary intervention should be guided by ischaemia-driven measurements and not solely by anatomical ones is growing.1,2 This is best demonstrated by a substudy of the Clinical Outcomes Utilising Revascularisation and Aggressive Drug Evaluation (COURAGE) trial, which showed that patients with the greatest relief of ischaemia by nuclear study had the lowest rates of death or myocardial infarction.21
QCA was developed to minimise inter-observer variability and to maximise reproducibility. It is well known that angiography is limited when evaluating coronary stenoses.22 Our study and others23,24 confirm that QCA has a limited ability to assess the functional severity of coronary stenosis. Further confirmation of this is provided by ameta-analysis of FFR versus QCA for evaluation of myocardial ischaemia. In that analysis, QCA did not predict the functional significance of coronary lesions.25 Thus, contrast angiography alone often does not provide sufficiently precise information about the haemodynamic consequences of an intermediate stenosis on which to base a definite decision regarding the need for revascularisation.
Computed tomography has not, to date, proven its value in this regard. Wijpkema et al26 reported no correlation between FFR and mean stenosis diameters calculated by computed tomography in patients scheduled for percutaneous coronary intervention; and Meijboom et al23 similarly reported no correlation between computed tomography and FFR in intermediate coronary artery stenoses. Kristensen et al,27 however, reported that in intermediate coronary stenoses, quantitative cross-sectional dimensions derived from 64-slice cardiac computed tomography significantly correlated with FFR. This was especially true of % area stenosis.
When considering whether it is realistic to expect a close correlation between MLA and FFR, it should be noted that MLA from IVUS examinations does not take into account differences in patient sizes and the size of individual coronary arteries. Consequently, the ability of MLA to reflect the physiological importance of given measurements might be compromised. This suggestion is plausible since the larger the myocardial mass subtended by a vessel, the greater the hyperaemic flow, and in turn, the larger the gradient and the lower the FFR. In this study, however, there was a significant correlation between FFR and % area stenosis (which does take variability in vessel size into account). Furthermore, we found a strong correlation between MLA and FFR in large vessels but not in medium-sized vessels. In addition, we found a different threshold for the MLA based upon the reference vessel diameter becoming smaller. Those with a small vessel and with MLA <4 mm2 can have FFR >0.8 and a large vessel with MLA <4 mm2 can have FFR <0.8.
IVUS and FFR are complementary techniques which have both been used in the catheterisation laboratory to provide critical anatomic and functional data regarding stenoses of intermediate severity. However, in the real world many center do not have both or are limited financially to use both for assessment same lesion. Virtually all physiological stenoses (as estimated by FFR <0.75) have an IVUS-derived MLA <4 mm2; yet many with that measurement do not. IVUS than MLA <4 mm2 in many cases especially in small vessel are not associated with ischaemia. Current evidence suggests that a stenosis with FFR <0.75 virtually always reflects potential ischaemia and that a measurement >0.75 with few exceptions excludes ischaemia related to that lesion. We found a moderately linear correlation between FFR and IVUS MLA. Our data further suggest that a MLA of 2.8 mm2 would be a better threshold for detecting FFR <0.75, while different thresholds should be used for different vessel sizes.
Conflict of interest statement
The authors have no conflict of interest to declare.

