
Abstract
Aims: In intermediate coronary artery disease, discordance between anatomical and functional assessments persists and the diagnostic accuracy of an anatomical evaluation is not satisfactory for determining functional significance. We aimed to evaluate the impact of microvascular resistance on “anatomical-functional discordance”.
Methods and results: In 97 intermediate coronary lesions of 83 patients, minimum lumen area (MLA), fractional flow reserve (FFR), ∆(Pd/Pa−FFR), and hyperaemic microvascular resistance index (hMVRI) were measured using intravascular ultrasound and an intracoronary dual pressure and Doppler sensor-tipped guidewire. hMVRI correlated with FFR and ∆(Pd/Pa−FFR) (r=0.611, p<0.001; r=−0.509, p<0.001; respectively). After the lesions were categorised into four groups based on functional significance (FFR 0.8) and the MLA cut-off for that (2.5 mm2), hMVRI was higher with a lower ∆(Pd/Pa−FFR) regardless of the MLA group in lesions with FFR >0.8, compared with those in lesions with FFR ≤0.8. hMVRI was independently associated with FFR and ∆(Pd/Pa−FFR) (β=0.443, p<0.001; β=−0.389, p<0.001; respectively).
Conclusions: Coronary microvascular resistance is associated with anatomical-functional discordance and the ischaemic potential of intermediate epicardial stenosis. In determining a treatment strategy, anatomy alone is insufficient and an integrated physiologic approach is important.
Abbreviations
CFR: coronary flow reserve
DS: diameter stenosis
FFR: fractional flow reserve
hMVRI: hyperaemic microvascular resistance index
IVUS: intravascular ultrasound
MLA: minimum lumen area
Introduction
Coronary revascularisation should be determined based on objective evidence of ischaemia to improve outcomes by avoiding potential risks related with unnecessary procedures, particularly in cases of intermediate coronary artery disease (CAD). Fractional flow reserve (FFR) has become the reference standard for invasively assessing the functional significance of epicardial stenosis including intermediate CAD after validation in landmark clinical trials1,2. To expand the clinical utility of anatomical measures of CAD and compensate for shortcomings in them, there has been much recent effort to integrate functional and anatomical assessments of coronary stenosis using FFR and coronary angiography (CAG) or intravascular imaging, such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT)3. However, “anatomical-functional” discordance remains, and the diagnostic accuracy of the minimum lumen area (MLA) is not satisfactory for determining the functional significance determined by FFR because other anatomical features also influence the ischaemic potential of a coronary lesion, although the MLA is the major determinant of the functional significance of a coronary stenosis4. In addition, impaired microvascular function may affect the functional significance of an epicardial stenosis manifesting as increased FFR by decreasing the translesional pressure gradient5. However, studies on the relationship between microvascular status and functional significance in intermediate CAD are lacking; thus, we evaluated the impact of microvascular resistance on the “anatomical-functional” discordance in intermediate epicardial lesions using invasive coronary physiologic measurements and IVUS. We hypothesised that discordance between FFR and IVUS is related to microvascular resistance despite similar anatomical features.
Methods
PATIENT POPULATION
We prospectively enrolled 83 patients between March 2013 and December 2014 who underwent intracoronary physiologic measurements and IVUS to evaluate 97 de novo coronary lesions with 40-70% diameter stenosis (DS) based on visual estimates of CAG. Patients with significant left main disease, graft vessel disease, two or more stenotic segments (>40% DS) in the same vessel, left ventricular ejection fraction (LVEF) <40%, presence of a collateral vessel, or contraindication to adenosine and those with infarct-related arteries were excluded. All patients provided written informed consent. This cross-sectional study was approved by the Institutional Review Board.
CAG AND INVASIVE PHYSIOLOGIC MEASUREMENTS
We performed CAG with a standard technique and the quantitative analysis using the Cardiovascular Angiography Analysis System II (CAAS II; Pie Medical, Maastricht, the Netherlands). The haemodynamic parameters were measured using a 0.014-inch dual pressure and Doppler sensor-tipped guidewire (ComboWire® XT; Volcano Corp., San Diego, CA, USA) with a recording device console (ComboMap®; Volcano Corp.). Maximal hyperaemia was induced with an intracoronary continuous infusion (240-360 μg/min) of adenosine via a microcatheter6.
The FFR was calculated as the ratio of mean distal coronary pressure (Pd) to mean aortic pressure (Pa) during maximum hyperaemia, and ∆(Pd/Pa−FFR) was defined as the difference between the baseline Pd/Pa and FFR. Coronary flow reserve (CFR) was calculated as the ratio of hyperaemic average peak flow velocity (APV) to baseline APV. The hyperaemic microvascular resistance index (hMVRI) was calculated as hyperaemic Pd divided by hyperaemic APV.
IVUS IMAGING AND ANALYSIS
IVUS was performed after administering an intracoronary bolus of 200 µg nitroglycerine using the Galaxy 2™ IVUS System (Boston Scientific, Marlborough, MA, USA), and a 40-MHz coronary imaging IVUS catheter (Atlantis™ SR Pro; Boston Scientific) was pulled back from the distal site through the target stenosis to the ostium using a motorised pullback device at 0.5 mm/s. The quantitative IVUS analysis was performed using computerised planimetry software (echoPlaque™ 3.0; INDEC Systems, Santa Clara, CA, USA), according to the American College of Cardiology Clinical Expert Consensus Document7.
LESION CLASSIFICATION
All lesions were categorised into four groups according to the IVUS-MLA cut-off value for functional significance (FFR ≤0.8) and FFR ≤0.8 or >0.8 to analyse further the relationship among functional severity, microvascular status, and anatomical stenosis: MLA(+)/FFR(+), MLA(+)/FFR(−), MLA(−)/FFR(+), and MLA(−)/FFR(−). The lesions were also stratified based on angiographic %DS ≥50% or <50% and the functional significance by FFR: %DS(+)/FFR(+), %DS(+)/FFR(−), %DS(−)/FFR(+), and %DS(−)/FFR(−).
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and percentages and compared using the chi-square or Fisher’s exact test. Continuous variables are presented as the mean±standard deviation or median with interquartile range according to their distribution and homogeneity, which was verified using the Kolmogorov-Smirnov test. The independent t-test and correlation analysis were used to compare continuous variables between two groups. Receiver operating characteristic (ROC) curve analysis was used to identify IVUS-MLA criteria to predict FFR ≤0.80. Since hMVRI was not normally distributed, log-transformed values of the original value (i.e., ln[hMVRI]) were created and used (Appendix Figure 1). Multivariate regression analyses identified the parameters independently associated with FFR, ∆(Pd/Pa−FFR) or functional significance (FFR ≤0.80). All of the variables with p<0.20, as determined by univariate analysis, and the factors that may potentially affect FFR (i.e., MLA, lesion length, hMVRI, age, lesion location, sex, diabetes, hypertension, dyslipidaemia, and smoking) were included in the multivariate model. SPSS, Version 23 (IBM Corp, Armonk, NY, USA) was used and a p-value <0.05 was considered significant.
Results
BASELINE AND ANGIOGRAPHIC CHARACTERISTICS
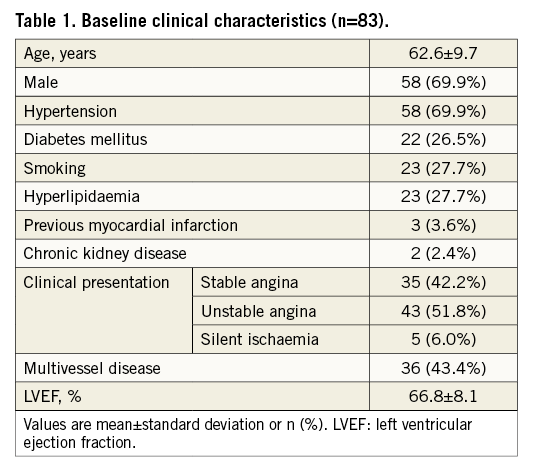
Table 1 and Table 2 present the baseline characteristics of the patients and lesions. The mean patient age was 62.6 years and 58 (69.9%) were male. Approximately 42% of the patients presented with stable angina and 43% of them had multivessel CAD. Sixty-seven lesions were in the left anterior descending artery (LAD), nine were in the left circumflex artery, and 21 were in the right coronary artery.
INTRACORONARY PHYSIOLOGIC MEASUREMENTS
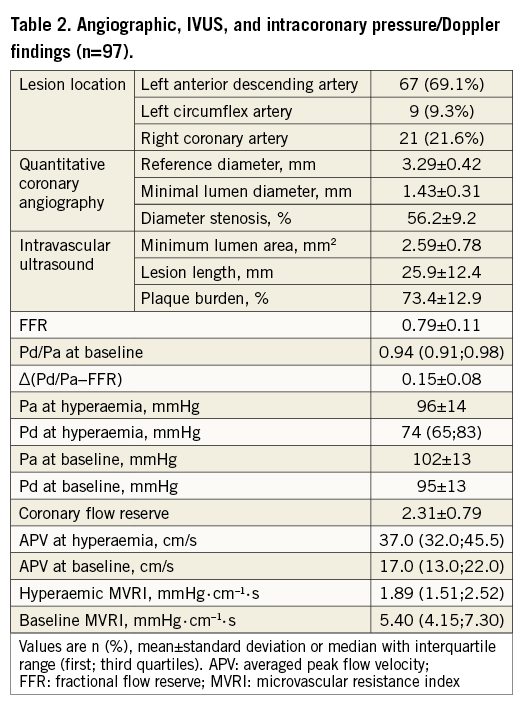
The mean FFR, ∆(Pd/Pa−FFR), CFR, and median hMVRI were 0.79±0.11, 0.15±0.08, 2.31±0.79, and 1.89 (1.51;2.52) mmHg·cm–1·s, respectively (Table 2). hMVRI was moderately but significantly positively correlated with FFR and negatively with ∆(Pd/Pa−FFR) (r=0.611, p<0.001; r=−0.509, p<0.001, respectively) (Figure 1). CFR weakly correlated with hMVRI (r=−0.221, p=0.030), but did not correlate with ∆(Pd/Pa−FFR) (Appendix Figure 2).
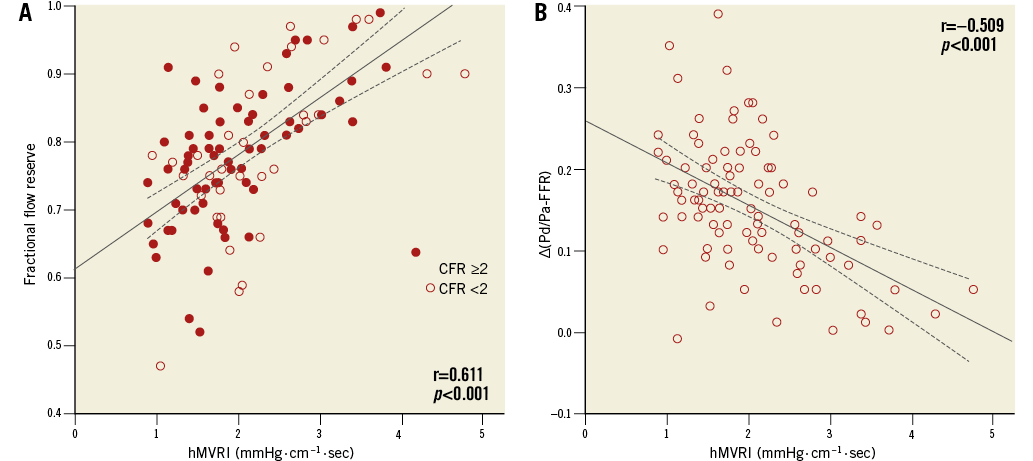
Figure 1. Correlations between the haemodynamic parameters. hMVRI demonstrated moderate correlations with FFR (A) and ∆(Pd/Pa−FFR) (B). CFR: coronary flow reserve; FFR: fractional flow reserve; hMVRI: hyperaemic microvascular resistance index.
RELATIONSHIP BETWEEN ANATOMY AND FUNCTIONAL SIGNIFICANCE
FFR was moderately correlated with MLA (r=0.446, p<0.001) and the best cut-off value of MLA for functional significance (FFR ≤0.8) was 2.49 mm2 (Figure 2).
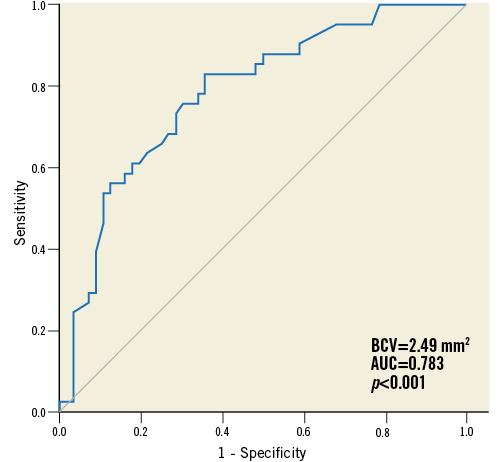
Figure 2. ROC curve analysis of MLA for predicting FFR ≤0.8. The best cut-off value for functionally significant stenosis was 2.49 mm2.
AUC: area under the curve; BCV: best cut-off value; MLA: minimum lumen area
In the concordant group, 36 (37%) lesions were included in the MLA(+)/FFR(+) group and 34 (35%) were in the MLA(−)/FFR(−) group. In the discordant group, seven (7%) lesions were included in the MLA(+)/FFR(−) group and 20 (21%) were in the MLA(−)/FFR(+) group. After the lesions had been stratified by an MLA of 2.5 mm2, hMVRI was compared between the high-FFR and low-FFR groups in lesions with similar MLA (Figure 3A). hMVRI was significantly higher in the high-FFR group than in the low-FFR group in lesions with MLA <2.5 mm2 (2.11±0.45 vs. 1.65±0.41, p=0.007) and MLA ≥2.5 mm2 (2.77±0.83 vs. 1.62±0.39, p<0.001). In lesions with FFR >0.8, ∆(Pd/Pa−FFR) was lower, regardless of MLA, compared to that in lesions with FFR ≤0.8 (0.13±0.06 vs. 0.20±0.07, p=0.020 in MLA <2.5 mm2; 0.09±0.06 vs. 0.19±0.04, p<0.001 in MLA ≥2.5 mm2). However, CFR was not different between the two FFR groups in either MLA group (2.36±0.59 vs. 2.32±0.85, p=0.931 in MLA <2.5 mm2; 2.30±0.77 vs. 2.30±0.82, p=0.990 in MLA ≥2.5 mm2). In addition, CFR did not correlate with MLA or %DS (r=−0.016, p=0.873; r=−0.016, p=0.873, respectively). The diagnostic accuracy of both MLA and %DS for CFR <2.0 was poor (Appendix Figure 3).
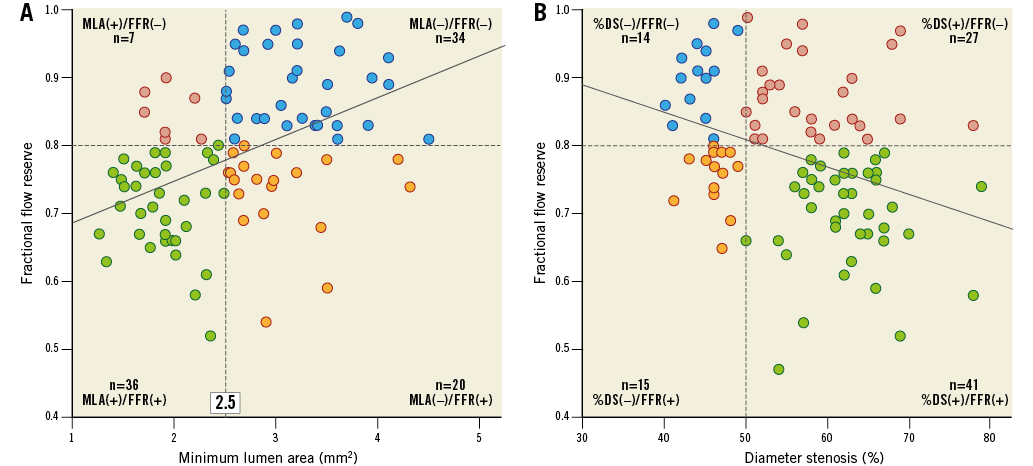
Figure 3. Functional and anatomical distribution of the lesions. A) The correlation between FFR and MLA. hMVRI was significantly higher in the high-FFR group than in the low-FFR group in both the large- and small-MLA groups. B) The correlation between FFR and %DS. hMVRI was significantly higher in the %DS(+)/FFR(−) group than in the %DS(+)/FFR(+) group. DS: diameter stenosis; FFR: fractional flow reserve; hMVRI: hyperaemic microvascular resistance index; MLA: minimum lumen area
Additional analysis of the discordance between FFR vs. CFR or Pd/Pa gave similar results. After dividing the lesions into either MLA group, hMVRI was compared between the high- and low-FFR groups in the FFR-CFR or FFR-Pd/Pa discordance groups. Similarly, hMVRI was higher in the high-FFR group than in the low-FFR group and the difference was significant in lesions with an MLA ≥2.5 mm2.
A weak but significant correlation was observed between FFR and %DS (r=−0.341, p=0.001) (Figure 3B). Similarly, hMVRI was significantly higher in the %DS(+)/FFR(−) group than in the %DS(+)/FFR(+) group (2.56±0.71 vs. 1.69±0.43, p<0.001). Also, patients with %DS(−)/FFR(−) had a higher hMVRI than those with %DS(−)/FFR(+) (2.78±0.99 vs. 1.51±0.31, p<0.001).
Mean FFR values were calculated in each group by tertiles of hMVRI and MLA to present FFR vs. hMVRI and MLA together (Figure 4). Both MLA and hMVRI showed positive and graded associations with FFR.
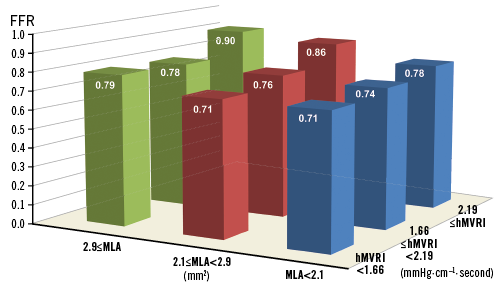
Figure 4. FFR vs. hMVRI and MLA. Mean FFR values were calculated in each tertile-hMVRI group and tertile-MLA group. Lesions with a higher MLA and higher hMVRI tended to have a higher FFR. FFR: fractional flow reserve; hMVRI: hyperaemic microvascular resistance index; MLA: minimum lumen area
INDEPENDENT DETERMINANTS OF FFR, ∆(Pd/Pa−FFR), AND FUNCTIONAL SIGNIFICANCE
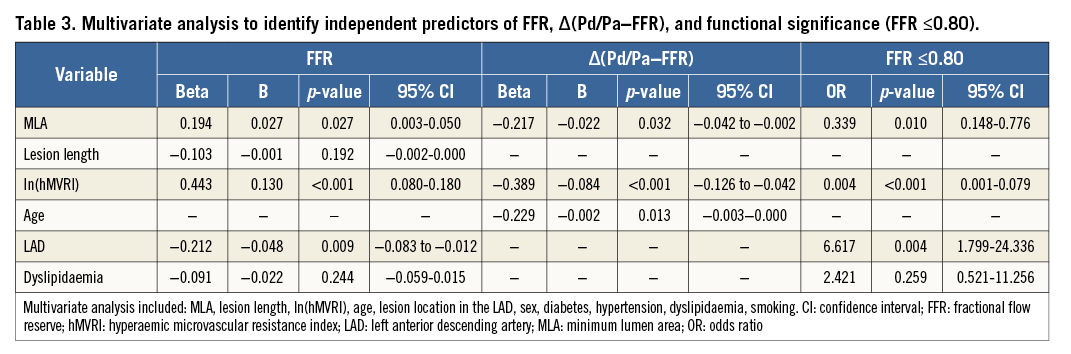
A multiple regression analysis showed that the independent determinants for FFR were MLA (β=0.194, p=0.027), lesion location in the LAD (β=−0.212, p=0.009), and ln(hMVRI) (β=0.443, p<0.001). The independent determinants for ∆(Pd/Pa−FFR) were MLA (β=−0.217, p=0.032), age (β=−0.229, p=0.013) and ln(hMVRI) (β=−0.389, p<0.001). A logistic regression analysis showed that the independent determinants for FFR ≤0.8 were MLA (odds ratio [OR]=0.339, p=0.010), ln(hMVRI) (OR=0.004, p<0.001), and lesion location in the LAD (OR=6.617, p=0.004) (Table 3).
Discussion
The main finding of this study was that discordance between the anatomical and functional significance of an intermediate epicardial stenosis is associated with microvascular function. The distinguishing feature of this study is that the impact of microvascular dysfunction on anatomical-functional discordance was evaluated in intermediate lesions using: 1) IVUS to overcome the limitations of CAG, and 2) a dual pressure and Doppler sensor-tipped guidewire to evaluate the functional significance of epicardial stenosis and microvascular resistance simultaneously8.
Coronary revascularisation including percutaneous coronary intervention (PCI) only for ischaemia-producing lesions is essential to improve clinical outcomes and avoid unnecessary procedures that lead to adverse events and procedure-related complications1,2. Therefore, objective evidence of inducible ischaemia for a patient with epicardial stenosis is important to determine the treatment strategy, particularly in intermediate stenoses where adjunctive, invasive evaluations are proposed frequently because of the highlighted limitations of CAG in this subset of coronary lesions9.
Measuring FFR is useful for detecting ischaemia-related coronary lesions and has been confirmed as providing useful guidance for determining treatment strategy in patients with intermediate CAD1,2. In addition, because of its higher spatial resolution, vascular wall imaging, and practical ease, IVUS has been widely used to determine lesion morphology and severity7,10. As MLA has been proposed as a simple anatomic alternative to determine functional severity of coronary narrowing, many studies have integrated the physiology and anatomy of a coronary narrowing by investigating the relationship between FFR and MLA using IVUS or even OCT3. However, discordance between the parameters of the two modalities is sizeable, and the diagnostic accuracy of MLA is not satisfactory to determine functional significance based on FFR.
Although IVUS-MLA was the most important determinant of functional significance of a coronary lesion, it did not account for other anatomical factors of the ischaemic potential of a coronary stenosis, such as lesion length and location, reference vessel diameter, lesion morphology, eccentricity, plaque burden, or amount of myocardium subtended by the lesion. In addition, several clinical and biological conditions, such as gender, age, diabetes, hypertension, and LVEF may affect the functional significance of an epicardial lesion11,12. A microvascular abnormality, by itself or as a major pathophysiology of the biological conditions mentioned above, could affect the ischaemic potential of an epicardial stenosis and is manifested by a higher FFR value because impaired microvascular function decreases the translesional pressure gradient5. We confirmed the independent association between microvascular dysfunction and the functional significance of epicardial stenosis by FFR, and the hypothesis that microvascular function was associated with the discordance between the anatomy and function of an intermediate epicardial lesion by demonstrating that lesions with higher microvascular resistance had higher FFR values despite similar anatomical characteristics compared to those of lesions with lower microvascular resistance. In addition, we found a negative correlation between microvascular resistance and ∆(Pd/Pa−FFR). Although ∆(Pd/Pa−FFR) is not a specific indicator of microvascular dysfunction, it reflects the microvascular compensatory response to an epicardial stenosis, and an inadequate drop in Pd/Pa related to impaired vasodilatation capacity of the microcirculation may lead to an elevated FFR13. In contrast with our results, Lee et al14 found no correlation between the index of microcirculatory resistance (IMR) and FFR. This can plausibly be explained as follows: compared with Lee et al, 1) our patients might be more likely to have had microvascular dysfunction because more unstable angina patients were included (52% vs. 26%); 2) our subjects had more severe coronary lesions, both morphologically and functionally (DS, 56% vs. 40%; FFR, 0.79 vs. 0.84); 3) in our study, there was a higher rate of LAD lesions (69% vs. 54%); and 4) the FFR distributions in the two studies are different, with our study showing a normal distribution (Appendix Figure 1) versus a distribution skewed to the non-ischaemic range.
Myocardial ischaemia can result not only from epicardial narrowing but also from other coronary circulatory pathology, such as microvascular abnormalities. In general, the microvascular abnormalities would not specifically affect the FFR-based clinical decision to use PCI. They might affect the ischaemic potential of a given epicardial stenosis to be restored by PCI and are manifested by higher FFR values. Nevertheless, our study is an important reminder that a dichotomous cut-off of 0.8 is not always an absolute criterion, while FFR is an adjunctive tool that provides supplementary information for the physician to determine the most appropriate treatment strategy. Along with the caution regarding the use of anatomy alone to determine a treatment strategy for intermediate CAD, there are also issues that need to be addressed regarding available physiologic indices, including FFR. Meuwissen et al15 reported that the hyperaemic stenosis resistance index, measured using pressure and flow velocity, was a more powerful predictor of reversible perfusion defects than CFR or FFR. Escaned et al16 also emphasised the combined use of pressure and flow velocity. Our study supports the theoretical evidence, which asserts that an increase in microvascular resistance decreases the trans-stenotic pressure gradient5, as we observed a “higher microvascular resistance−higher FFR” relationship. Although the clinical usefulness of FFR is not compromised (except in cases with severe microvascular dysfunction), it may be important to take an integrated physiologic approach for patients in whom small changes in FFR may alter the treatment decision (e.g., patients with intermediate lesions with borderline FFR). The results of our study also suggest that active evaluation and treatment of microvascular dysfunction is required in patients with intermediate CAD. Particularly for negative FFR notwithstanding a tight stenosis, microvascular dysfunction needs to be actively measured while performing additional non-invasive stress examinations. These may lead to the improved identification of treatment targets for inducible ischaemia in the subtended myocardium. This strategy is supported by a recent study reporting a 21% incidence of microvascular dysfunction, even in patients without obstructive CAD17. Integrating pressure, flow, and resistance (rather than FFR alone) may help to provide a more comprehensive overview of the coronary status for clinical decision making.
In this regard, concerning the potential for microvascular dysfunction and the subsequent subhyperaemic response to adenosine, the instantaneous wave-free ratio (iFR) may be advantageous. When iFR, FFR, and CFR were compared, iFR agreed more closely with CFR18, suggesting that it has a stronger association with both hyperaemic flow velocity and CFR than FFR.
Interestingly, CFR was similar between lesions with FFR ≤0.8 and FFR >0.8 in both IVUS-MLA groups in our study. This suggests that, if a patient has microvascular dysfunction, a tighter epicardial stenosis is needed to induce myocardial ischaemia from the epicardial vessel; however, the results should be interpreted with caution and further studies are needed. Despite the limitation due to the small number of subjects in our study and the difficulty presenting a clear explanation for why the CFR values were >2 in all four groups, a plausible mechanism is that both the hyperaemic and baseline APV may decrease when the microvascular resistance increases. Consequently, CFR might not change significantly. By contrast, hMVRI may increase because APV decreases while Pd increases during hyperaemia when microvascular resistance increases. Our hypothesis is supported by the increase in the resting coronary blood flow and subsequent decrease as the extent of coronary microembolisation is increased19, and that CFR is preserved despite a high IMR since the resting mean transit time is higher in lesions with a high CFR and high IMR compared to other groups20. In addition, hMVRI was higher in the high-FFR group than in the low-FFR group, regardless of the CFR or Pd/Pa groups. These results correspond to a previous study21 and our findings showed the correlation between hMVRI and ∆(Pd/Pa−FFR). Predictably, hMVRI might be highest in cases with a high FFR and low CFR or a high FFR and low Pd/Pa. Therefore, it would be better to measure both pressure and flow velocity to obtain a deeper understanding of the haemodynamics of microvascular resistance.
Limitations
This study has limitations. First, if a reference examination of myocardial ischaemia (i.e., ammonia-positron emission tomography or stress perfusion magnetic resonance imaging) was available as a confirmatory test, we could provide more informative findings regarding the relationship between anatomical-functional discordance, microvascular resistance, and the ischaemic potential of epicardial stenosis. Second, viability of the subtended myocardium was not evaluated even though viability can affect FFR. However, only 3.6% of the patients in this study suffered from old or acute myocardial infarction, and infarct-related arteries were excluded. Therefore, we believe that this is not a critical limitation. Third, we could not account for collateral flow when measuring microvascular resistance. This might be irrelevant for FFR values above 0.80, since little collateral flow would be expected. However, the microvascular resistance was probably overestimated in those lesions that had lower FFR values, although this would not be expected to change the results.
Conclusions
Considerable discordance was observed between anatomical and functional evaluations in intermediate epicardial stenosis. Microvascular resistance of the coronary circulation was shown to be associated with anatomical-functional discordance and the ischaemic potential of intermediate epicardial stenosis by pressure-based evaluation. Therefore, anatomy alone is insufficient to determine treatment strategy, and an integrated physiologic approach is required.
| Impact on daily practice In intermediate epicardial stenosis, the discordance between anatomical and functional significance is sizeable and is associated with microvascular function. Therefore, determining treatment strategy by anatomy alone is insufficient. In addition, there is a need for a physiologic approach that integrates pressure, flow, and resistance. This type of approach would be particularly useful for lesions determined to be of borderline functional significance by pressure-based evaluation due to the potential for a relationship between FFR and microvascular dysfunction. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Appendix Figure 1. Distribution of FFR, hMVRI and ln(hMVRI). FFR: fractional flow reserve; hMVRI: hyperaemic microvascular resistance index; ln(hMVRI): log-transformed value of the original value
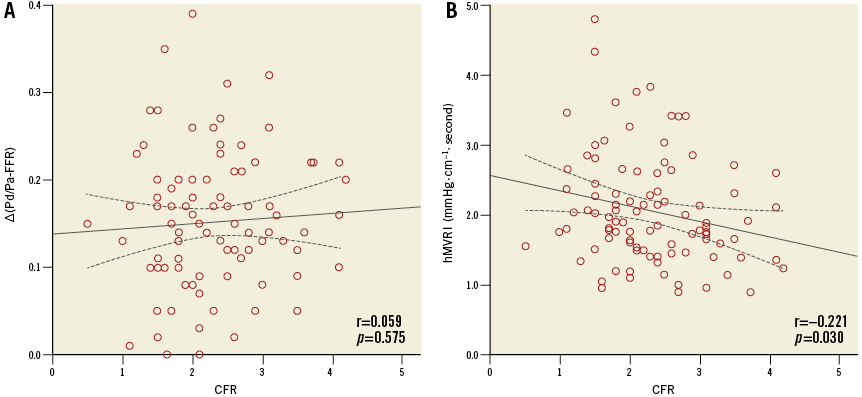
Appendix Figure 2. Correlation curves. Correlation between CFR, ∆(Pd/Pa−FFR) (A) and hMVRI (B). CFR: coronary flow reserve; FFR: fractional flow reserve; hMVRI: hyperaemic microvascular resistance index.
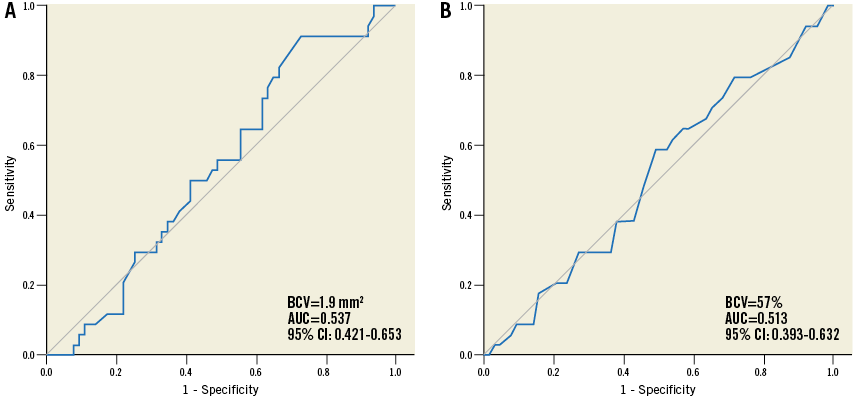
Appendix Figure 3. Receiver-operating characteristic curve criteria to predict a CFR <2.0. IVUS-MLA (A) and %DS (B). BCV: best cut-off value

