Abstract
Aims: The influence of atherosclerosis and its risk factors on coronary microvascular function remain unclear as current methods of assessing microvascular function do not specifically test the microcirculation in isolation. We examined the influence of epicardial vessel atherosclerosis on coronary microvascular function using the index of myocardial resistance (IMR).
Methods and results: IMR (a measure of microvascular function) and fractional flow reserve (FFR, a measure of the epicardial compartment) were measured in 143 coronary arteries (116 patients). Fifteen patients (22 arteries, mean age 48±16 years) had no clinical evidence of atherosclerosis (control group). One hundred and one patients (121 arteries, mean age 63±11 years) had established atherosclerosis and multiple cardiovascular risk factors (atheroma group). Mean IMR in the control group (19±5, range 8-28) was significantly lower than in the atheroma group (25±13, range 6-75) (P<0.01). However, there was large overlap between IMR in both groups, with 69% of IMR values in patients with atheroma being within the control range. Mean FFR was also higher in the control group (0.96±0.02, range 0.93-1.00) than in the atheroma group (0.85±0.14, range 0.19-1.00) (P<0.01). There was no correlation between IMR and FFR (r=0.09; P=0.24), even when results in the control (r=0.02; P=0.92) and atheroma (r=0.15; P=0.10) groups were analysed in isolation. Using stepwise multiple regression analysis presence/absence of atheroma (ß=0.42; P=0.02) was the only independent determinant of IMR.
Conclusions: Mean IMR is higher in patients with epicardial atherosclerosis. However, there is a large overlap between IMR in patients with and without epicardial atherosclerosis.
Introduction
A number of animal studies have shown that the biological effects of atherosclerosis also extend into the coronary microcirculation1-4. Although, there are no direct in vivo studies investigating the influence of atherosclerosis on the human coronary microcirculation, it is generally assumed that microvascular dysfunction is also present in subjects with epicardial atherosclerosis5,6.
It has been proposed that assessment of coronary microvascular function may be important in risk stratification and determination of prognosis in cardiac patients5-12. Functional information on the state of the coronary microcirculation is currently derived from quantification of blood flow through the coronary circulation5. However, accurate assessment of the coronary microcirculation, especially in the context of epicardial vessel atherosclerosis, remains difficult as most currently available methods simultaneously interrogate both the epicardial and microvascular compartments of the coronary vascular bed13,14. Focal epicardial vessel stenosis as well as diffuse atherosclerosis in the absence of focal stenosis can both impair myocardial / epicardial blood flow15. Consequently, methods that determine myocardial blood flow from systemic pressure, instead of distal coronary pressure, are not suited to specifically investigate coronary microvascular function in individuals with epicardial atherosclerosis.
The index of myocardial resistance (IMR) has recently been introduced and validated as a measure of coronary microvascular resistance and thus as an alternative marker of microvascular function16-18. It can easily be obtained in the catheterisation laboratory with a commercially available, combined, pressure/temperature guidewire. IMR is calculated from the ratio of the distal coronary pressure and the inverse of mean transit time (Tmn) of a bolus of saline injected through a coronary guide catheter during maximal hyperaemia16. Therefore, in contrast to other methods for assessing coronary microcirculation, IMR is specific to the microvascular compartment. IMR has been shown to correlate closely with absolute microvascular resistance and is not influenced by systemic haemodynamics, baseline coronary flow or resistance15-19.
The purpose of this study was to investigate the influence of epicardial vessel atherosclerosis on coronary microvascular function as assessed by the IMR.
Methods
Study population
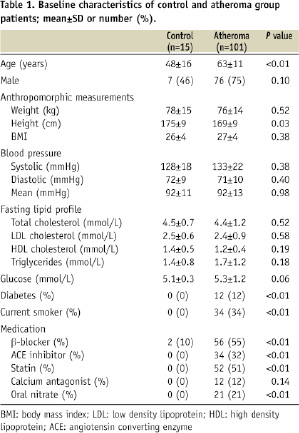
One hundred and forty-three coronary arteries were investigated in 116 patients. The ‘control’ group consisted of 15 patients (in whom 22 coronary arteries were studied) with no clinical evidence of coronary atherosclerosis. These patients underwent cardiac catheterisation for non-coronary reasons: seven patients prior to closure of a patent foramen ovale and eight patients prior to electrophysiological examination for cardiac arrhythmia. Control patients never reported chest pain and had no or minimal risk factors for coronary artery disease. At coronary angiography, all control patients had perfectly normal, smooth coronary arteries. The ‘atheroma’ group consisted of 101 patients (in whom 121 coronary arteries were studied) with clinical evidence of coronary atherosclerosis undergoing elective pressure wire assessment of at least one epicardial vessel. Most patients had multiple cardiovascular risk factors (Table 1).
In 27 patients (seven from the control group and 20 from the atheroma group), microvascular function was assessed in two different vascular territories. There was no left ventricular (LV) wall motion abnormality in the myocardial territory supplied by the study vessel, and none of the subjects had echocardiographic evidence of LV hypertrophy. Patients with >50% angiographic evidence of left main stem coronary disease, unstable coronary syndrome, previous coronary artery bypass surgery, LV ejection fraction <50%, impaired RV function, elevated pulmonary pressures and valvular heart disease, were excluded. The study had local ethics committee approval and all patients gave informed consent prior to recruitment to the study.
Study protocol
Each patient underwent detailed characterisation on the basis of anthropomorphic measurements (height [m], weight [kg], body mass index [BMI, kg/m2]), fasting lipid profile (low density lipoprotein [LDL] cholesterol, high density lipoprotein [HDL] cholesterol and triglyceride concentrations [all in mmol/L]), fasting glucose concentration (mmol/L) and cardiac catheterisation for assessment of LV and coronary physiological indices (Fractional Flow Reserve [FFR] and IMR). Studies were performed in the morning after an overnight fast. Anthropomorphic measurements and venesection for biochemical analysis was performed immediately prior to cardiac catheterisation. All vasoactive medication was discontinued ≥ 24 hours before study measurements.
Cardiac catheterisation protocol
Each patient initially underwent standard selective coronary and LV angiography to delineate coronary anatomy and assess LV volumes and % ejection fraction. Weight-adjusted unfractionated heparin (100 U/kg) was administered during these procedures.
Coronary physiological indices were derived in each study artery using previously described principles and methods16-23. FFR was calculated from the ratio of distal coronary pressure (Pd) to proximal coronary pressure (Pa) at maximal hyperaemia20. In study vessels without a haemodynamically significant epicardial stenosis (FFR >0.75) and collaterals (125 study arteries), IMR was calculated from the ratio of mean distal coronary pressure at maximal hyperaemia to the inverse of the hyperaemic Tmn16,17. However, in vessels with a significant epicardial stenosis (FFR ≤ 0.75), Tmn may no longer be proportional to true myocardial blood flow, as it may not account for the potential contribution of the collateral circulation to myocardial blood flow18,19. In all such study vessels (18 arteries), IMR was calculated using the following equation, IMR=PaTmn [(Pd–Pw) / (Pa–Pw)] (Pw=coronary wedge pressure), to account for the influence of the collateral circulation18,19. FFR and IMR were measured in one or two coronary vessel(s) in each study patient.
A 6 Fr guide catheter was used to intubate coronary ostia. An intracoronary combined pressure/temperature sensor-tipped guidewire (Radi Pressure Wire® 5, Radi Medical Systems, Uppsala, Sweden) was used to measure distal coronary pressure and to derive thermodilution curves. After calibration, the Pressure Wire® was advanced to the tip of the guide catheter for equalisation of pressure and temperature signals and then positioned approximately 10 cm distal to the ostium of the study artery. All lines were flushed with normal saline to avoid injection of contrast medium prior to baseline measurements. 200 µg of intracoronary isosorbide dinitrate was administered prior to any measurement. Thermodilution curves were obtained (in triplicate) from a brisk, hand-held, 3-4 ml injection of room temperature saline at baseline and at maximal hyperaemia (Figure 1).
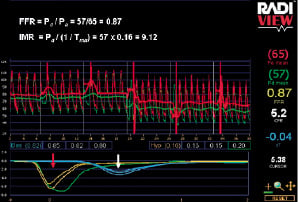
Figure 1. Simultaneous assessment of FFR and IMR. Mean transit times (Tmn) in triplicate are recorded at baseline (white arrow) and maximal hyperaemia (red arrow), as well as proximal (Pa) and distal (Pd) coronary pressures. FFR is derived from the ratio of Pd and Pa during maximal hyperaemia. IMR is derived from the ratio of mean distal coronary pressure (Pd) at maximal hyperaemia to the inverse of the hyperaemic Tmn.
Maximal hyperaemia was achieved either by infusion of adenosine via the femoral vein (140 µg/kg/min; in 114 study arteries), or from bolus injection of intracoronary papaverine (15 mg in the left coronary artery and 10 mg in the right coronary artery; in 29 study arteries)24. Mean Tmn at baseline and maximal hyperaemia were derived on-line from thermodilution curves within the Radi-Analyser unit. Simultaneous recordings of mean aortic pressure (from the guide catheter [Pa]) and mean distal coronary pressure (from the distal pressure / temperature sensor [Pd]) were also made at baseline and maximal hyperaemia. Subsequent to these measurements, routine percutaneous coronary intervention (PCI) was performed in study arteries with FFR ≤0.75. The Pressure Wire® was used as a PCI guidewire. During PCI, the distal coronary pressure (from the distal pressure/temperature sensor) measured during complete balloon occlusion of the vessel was taken as the coronary wedge pressure.
Statistical analysis
Data analysis was performed with SPSS 15.0 for Windows statistics software. Continuous variables were presented as mean±SD. Unpaired t test was used to analyse differences in continuous variables. Chi squared test was used to analyse differences in categorical variables. Pearson’s correlation coefficients (two-sided) were derived to assess the relationship between IMR and clinical / biochemical variables. A multivariate regression model was produced to identify independent determinants of IMR (covariates included in the model were age, gender, smoking history, BMI, mean arterial pressure, fasting lipid profile [LDL / HDL cholesterol and triglyceride concentration], fasting glucose concentration, % LV ejection fraction, FFR and presence / absence of epicardial vessel atherosclerosis). Bonferroni correction was applied to results to correct for multiple analyses. All variables were selected a priori. In line with previous publications the mean variability between triplicates of Tmn was 16% at baseline and 15% at maximal hyperaemia (P=0.53)22,23. Statistical significance was accepted at P<0.05.
Results
Baseline characteristics of patients are outlined in Tables 1 and 2. The control group was younger (P<0.01) and had very few risk factors for atherosclerosis in comparison to the atheroma group. There were no differences in % LV ejection fraction and other physiological parameters between the two groups. In all study arteries with a FFR ≤0.75 (18 study arteries, mean FFR 0.60±0.17), coronary wedge pressure was also measured to account for the potential influence of the collateral circulation on IMR.
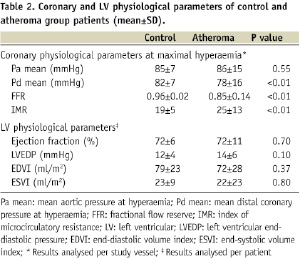
IMR and FFR values in the presence and absence of atherosclerosis
Mean IMR in patients with clinical atherosclerosis was significantly higher than in patients without atherosclerosis (atheroma group: 25±13 [range 6 to 75], control group: 19±5 [range 8 to 28]; P<0.01) (Figure 2). However, there was a large overlap, with 69% of IMR values in the atheroma group being in the control range (Figure 2). All IMR values in the control group were <30. As expected mean FFR values were higher in the control group (atheroma group: 0.85±0.14 [range 0.19 to 1.00], control group: 0.96±0.02 [range 0.93 to 1.00]; P<0.01) (Figure 2).
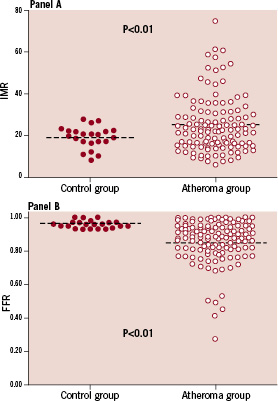
Figure 2. Distribution of IMR values (panel A) and FFR values (panel B) in control and atheroma patients.
There was no correlation between IMR and FFR (r=0.09; P=0.24) (Figure 3), even when results in the control (r=0.02; P=0.92) and atheroma (r=0.15; P=0.10) groups were analysed in isolation.
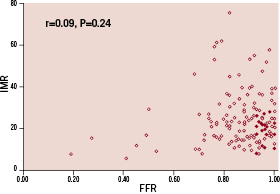
Figure 3. Correlation between IMR and FFR in patients with documented epicardial atherosclerosis and controls (denotes patients with epicardial vessel atherosclerosis and denotes patients with out atherosclerosis)
In seven of the control and 20 of the atheroma group patients, IMR was measured in two coronary arteries in each patient. There were no differences in mean IMR between the first and second measurements in each patient for both the control (artery one: 21±2, artery two: 17±4; P=0.10) and atheroma (artery one: 26±10, artery two: 30±12; P=0.26) groups.
Clinical determinants of IMR
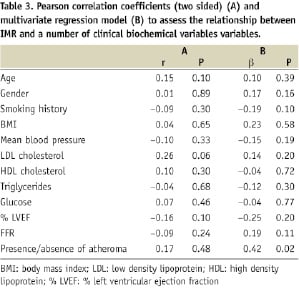
Pearson’s correlation coefficients for the association between IMR and clinical variables is summarised in Table 3. There was no significant correlation between any of the clinical / biochemical variables and IMR. A stepwise multivariate regression model was produced to identify independent determinants of IMR. The presence / absence of atheroma (P=0.02) was the only independent determinants of IMR (Table 3).
Discussion
Using IMR as a specific marker for coronary microvascular resistance16-19, we studied the association between the presence / absence of epicardial atherosclerosis and microvascular function. Our results suggest that, 1) mean IMR is higher in patients with epicardial atherosclerosis and, 2) there is a large overlap between IMR in patients with and without documented epicardial atherosclerosis.
Coronary microvascular function in subjects with and without atherosclerosis
Although mean IMR is higher in patients with documented epicardial atherosclerosis (atheroma group), there is significant overlap with IMR in patients with no epicardial evidence of atherosclerosis and minimal cardiovascular risk factors (control group). This finding suggests that in contrast to general belief epicardial vessel atherosclerosis is not uniformly accompanied by microvascular dysfunction.
In patients with coronary disease, as investigated in our study, FFR can be taken as an indirect marker for atherosclerotic burden as FFR has been shown to correlate with intravascular ultrasound (IVUS) 3D quantification of epicardial atherosclerosis25,26. Therefore, the absence of correlation between FFR and IMR values in arteries with a wide range of atherosclerotic changes, as in our study, is further evidence suggesting that the presence of epicardial atherosclerosis is not always associated with abnormal microvascular resistance. Recent data from Lavi et al also suggest the there may be a functional dissociation between coronary epicardial and microvascular compartments27. In their study of young smokers, epicardial vessel endothelial dysfunction was not uniformly accompanied by microvascular endothelial dysfunction in all coronary arteries27.
IMR as a specific marker of coronary microvascular function
To date the majority of studies investigating coronary microcirculatory function have used the principles of flow reserve to measure microvascular resistance1,13,14,27,28. Using this principle, multiple invasive and non-invasive studies in humans and animal models of microvascular dysfunction have demonstrated a reduction in flow reserve in the context of classic cardiovascular risk factors (such as smoking, hypertension, diabetes and abnormal lipid profile), aortic valve stenosis, hypertrophic cardiomyopathy, as well as experimental animal models of atherosclerosis1,13,14,28. However, an important limitation of these studies has been the inability to distinguish between the relative contribution of the epicardial and microvascular compartments towards changes in flow reserve.
It has been shown in patients with diffuse epicardial atherosclerosis that the distal coronary pressure during maximal hyperaemia is often (and unpredictably) lower than central aortic pressure15. This observation indicates that the resistance conferred by diffuse atherosclerotic changes in epicardial coronary vessels is not negligible, even when no focal significant stenosis is seen on selective coronary angiography. The present study confirms these findings, as in approximately two third of patients with diffuse epicardial atherosclerosis FFR in the distal part of the artery was lower than the lowest value observed in the control group. These findings also imply that assessment of microvascular resistance using distal coronary pressure is potentially more accurate than the use of central aortic pressure to derive myocardial blood flow as used when deriving flow reserve. The use of central aortic pressure will neglect the epicardial contribution to total coronary resistance / changes in coronary blood flow and thus overestimate true microvascular resistance.
Previous studies investigating normal and impaired IMR values have been confined to experimental animal models. In an open chest porcine model, Fearon et al demonstrated that IMR values increase significantly in response to mechanical disruption of the coronary microcirculation16. Our results confirm for the first time that patients with atherosclerosis have higher mean IMR values in comparison to controls, indicating microvascular dysfunction. However, the range of values obtained in human coronary arteries in our study was greater than in previous experimental models. The overall state of the coronary microcirculation is dependent on the relative function of the endothelium dependent and independent components of the microvascular bed5. It is likely that in the acute state mechanical disruption of the microcirculation in experimental models may only have influenced the endothelium-independent component of the microcirculation. This may have contributed to the lower variability in IMR values observed in experimental models. In addition, unlike our study, previous animal experimental models investigating IMR were performed under general anaesthesia, a potential confounding factor.
IMR range and microvascular function
An important current limitation of IMR is the absence of a cut-off value to indicate microvascular dysfunction. Our data addresses this question by establishing the upper limit for thermodilution-derived IMR in young subjects without clinical evidence for microvascular dysfunction and/or atherosclerosis (control group). In 22 coronary arteries of subjects with no history of chest pain and angiographically smooth epicardial vessels (FFR >0.93 in all study arteries), all IMR values were below 30. However, these results must be interpreted with caution, as microvascular dysfunction may exist in the absence of established atherosclerosis and/or cardiovascular risk factors. Corroborating evidence for microvascular function using alternative investigative modalities such as cardiac magnetic resonance imaging (MRI) or positron emission tomography (PET) in this group of patients will further confirm our preliminary findings. Furthermore, possible future studies should ideally examine coronary microcirculation in a larger cohort of patients to encompass potential variations in microvascular function.
Independent determinant of coronary microvascular function
Using multivariate analysis the presence / absence of atheroma was the only independent determinant of IMR. Unlike previous studies of coronary microvascular function on multivariate analysis individual classical cardiovascular risk factors were not independent determinants of IMR in our study. This observation may be reconciled by the suggestions that a non-specific hyperaemic agent such as adenosine acts primarily on microvascular smooth muscle cells, which are more likely to be influenced directly by the presence of atherosclerosis as apposed to individual cardiovascular risk factors29. In addition, despite significant differences in age between the control and atheroma groups, on multivariate analysis age was not an independent determinant of IMR. It is unknown whether age influences the ‘normal’ range for microvascular function, thus further studies are required to directly examine the influence of age on coronary microvascular function.
Increased LV mass, as seen in patients with LV hypertrophy, is an important cause of impaired microvascular function9. Although, LV mass was not measured directly in this study all patients with echocardiographic evidence of LV hypertrophy were excluded to minimise the influence of increased LV mass on coronary microvascular function.
Homogeneity of the microvascular function
There is ongoing debate as to whether coronary microvascular function is uniformly distributed throughout the myocardium, or whether there are regional differences in microvascular function in each of the three main coronary arterial vascular territories30. Our study suggests that in the absence of wall motion abnormalities, microvascular resistance appears to be similar in two different vascular territories in controls, as well as in patients with atherosclerosis. This observation is suggestive that systemic determinants of microvascular function, such as atherosclerosis, are likely to have a uniform influence on the coronary microcirculation in each individual. However, our sample size was small and it is possible that this observation may be secondary to a type II error.
Study limitations
IMR is a specific marker of coronary microvascular resistance. Unlike other indices of coronary microvascular function its derivation incorporates the true driving pressure for myocardial blood flow. However, both IMR and other indices of microvascular resistance share an important conceptual limitation, namely myocardial blood flow. Myocardial blood flow is determined by myocardial resistance, which in turn is influenced by the mass of tissue perfused in each arterial territory. Considering the myocardial distribution of each coronary artery is unknown and variable from patient to patient, this may potentially explain the large variability of ‘control’ IMR values obtained in our study. In order to reduce any variability in IMR introduced as a result of methodological differences in measuring flow, we standardised the distance between the distal end of the guide catheter and the pressure / temperature sensor. On the basis of these considerations the ideal index for assessment of coronary microvascular resistance should be derived from the combination of distal coronary driving pressure (as obtained for IMR in the present study) and absolute myocardial blood flow expressed per gram of myocardial tissue (as can be obtained by PET).
A combination of central infusion of adenosine and bolus intra-coronary injection of papaverine, were used to achieve maximal hyperaemia in our patients. Ideally a single agent should have been used to achieve hyperaemia to avoid potential variability in the extent of hyperaemia achieved. However, previous studies have demonstrated that the dosage and mode of administration of both adenosine and papaverine, as used in our study, are capable of inducing maximal hyperaemia for an adequate period of time to ensure assessment of both FFR and thermodilution-derived IMR24. Furthermore, there was no difference in the variability of maximal hyperaemic Tmn values in triplicate between adenosine and papaverine (data not shown). In addition, there is ongoing debate, as discussed earlier, whether non-specific agonists such as adenosine / papaverine comprehensively interrogate both the endothelium-dependent and -independent components of the coronary microcirculation29.
Conclusions
The present data indicate that although mean microvascular resistance in patients with epicardial vessel atherosclerosis is higher than in controls, there is a large overlap between the two groups. In addition, our results further suggest that in subjects with no clinical evidence for microvascular dysfunction and/or atherosclerosis (control subjects) the range of IMR values is narrow and potentially confined to less than 30.
Acknowledgements
This work was supported by the Meijer Lavino Foundation for Cardiac Research. During the period of this study, Narbeh Melikian was John Parker British Cardiac Society Research Fellow at the Cardiovascular Division, King’s College London School of Medicine, London, United Kingdom.

