Abstract
Aims: The aim of this study was to test the hypothesis that coronary microvascular endothelial dysfunction (CMED) is associated with epicardial coronary atherosclerosis.
Methods and results: We performed a cross-sectional analysis of a comprehensive invasive assessment of coronary physiology with a focus on endothelium-dependent coronary microvascular function and virtual-histology intravascular ultrasound (VH-IVUS) in a total of 148 consecutive patients with chest pain and angiographically normal coronary arteries or non-obstructive coronary artery disease (CAD). Endothelium-dependent coronary vascular reactivity was evaluated by graded doses of intracoronary acetylcholine (ACh). CMED was defined as a percent increase in coronary blood flow of ≤50% in response to ACh. Patients with CMED (n=87) showed more vulnerable plaque characteristics as compared to those without (n=61); they showed higher plaque burden in association with larger necrotic core volume and higher frequency of imaged arteries containing at least one VH-IVUS-derived thin-capped fibroatheroma (TCFA) (n=22 [25.3%] vs 5 [8.2%], p=0.008). Multivariate logistic regression analysis revealed that CMED was an independent predictor of VH-IVUS-derived TCFA (adjusted odds ratio 2.28 [95% confidence interval: 1.30-4.02], p=0.004).
Conclusions: Independently of conventional coronary risk factors, CMED was associated with vulnerable plaque characteristics in patients with non-obstructive CAD.
Introduction
Accumulating evidence has demonstrated that endothelial function plays essential roles in maintaining vascular homeostasis in health and disease and that endothelial dysfunction is the hallmark of atherosclerotic cardiovascular diseases1. We have previously demonstrated that epicardial coronary endothelial dysfunction is associated with the development and progression of the local coronary atherosclerotic lesion in patients with chest pain and non-obstructive coronary artery disease (CAD)2,3. Although functional and structural abnormalities of epicardial coronary arteries have been the focus of previous studies, those of coronary microvasculature have gained increasing attention in many clinical settings4,5,6. Previous studies have shown that patients with non-obstructive CAD with concomitant coronary microvascular endothelial dysfunction (CMED) are, as compared to those without CMED, associated with increased cardiac events including myocardial infarction, percutaneous or surgical revascularisation, and cardiac death7,8. Moreover, the prevalence of CMED in this clinical entity has been shown to be not negligible6,9. A comprehensive invasive assessment of coronary endothelial function is feasible and of diagnostic value to detect patients with CMED4,6,9.
However, the role of CMED in the early stage of coronary atherosclerosis is largely unknown. The aim of the present study was to test the hypothesis that CMED is associated with epicardial atherosclerotic CAD. We performed a cross-sectional analysis over a decade of a comprehensive invasive assessment of coronary physiology with a focus on endothelium-dependent coronary microvascular function and virtual-histology intravascular ultrasound (VH-IVUS) in patients with chest pain and non-obstructive CAD.
Methods
More detailed methods are available in Supplementary Appendix 1.
STUDY DESIGN AND POPULATION
This retrospective, single-centre, cross-sectional study was performed at the Mayo Clinic in Rochester, MN, USA, using a prospective database of the Mayo Clinic Cardiac Catheterization Laboratory. Between July 2005, when VH-IVUS data became available, and February 2018, consecutive patients with angiographically normal coronary arteries or non-obstructive CAD who underwent both invasive coronary endothelial function testing and VH-IVUS examination in the left anterior descending coronary artery (LAD) for evaluation of chest pain were enrolled. Inclusion and exclusion criteria were as described previously7,9,10. The study population minimally overlapped with previous publications2,9,10.
COMPREHENSIVE INVASIVE ASSESSMENT OF CORONARY PHYSIOLOGY
Invasive assessment of endothelium-dependent and endothelium-independent coronary vascular responses in the LAD was performed in a comprehensive manner using a 0.014-inch Doppler guidewire (FloWire®; Philips Volcano, Rancho Cordova, CA, USA), as described previously2,7,11. Endothelium-independent coronary flow reserve (CFR) was obtained at maximum hyperaemia induced by intracoronary bolus injections of adenosine at incremental doses (18 to 72 µg). A CFR value of <2.0 was considered abnormal12,13. Endothelium-dependent coronary vascular reactivity was assessed by selective infusion of incremental doses of acetylcholine (ACh) into the LAD at 10–6, 10–5, and 10–4 mol/L2,7,11. Coronary blood flow (CBF) was calculated by the following equation: CBF=≠(time-averaged peak velocity)(coronary artery -diameter/2)2,14. CMED was defined as a percent increase in CBF of ≤50%, and epicardial coronary endothelial dysfunction as a percent decrease in coronary artery diameter of ≥20%, from baseline in response to a maximum dose of ACh2,7,11. To examine the dose-effect relationship, CMED was further subclassified into mild (percent change in CBF in response to a maximum dose of ACh of ≤50% but ≥0%) or severe (that of <0%)7.
GREYSCALE AND VIRTUAL-HISTOLOGY INTRAVASCULAR ULTRASOUND
Greyscale and VH-IVUS images were obtained and analysed as described previously2,3,15. VH-IVUS-derived thin-capped fibroatheroma (TCFA) was defined as plaque burden of >40% with confluent necrotic core of >10% in contact with the vessel lumen on at least three consecutive VH-IVUS frames13,16,17. The high accuracy of VH-IVUS-derived tissue characterisation has been histologically validated in humans18,19.
STATISTICAL ANALYSIS
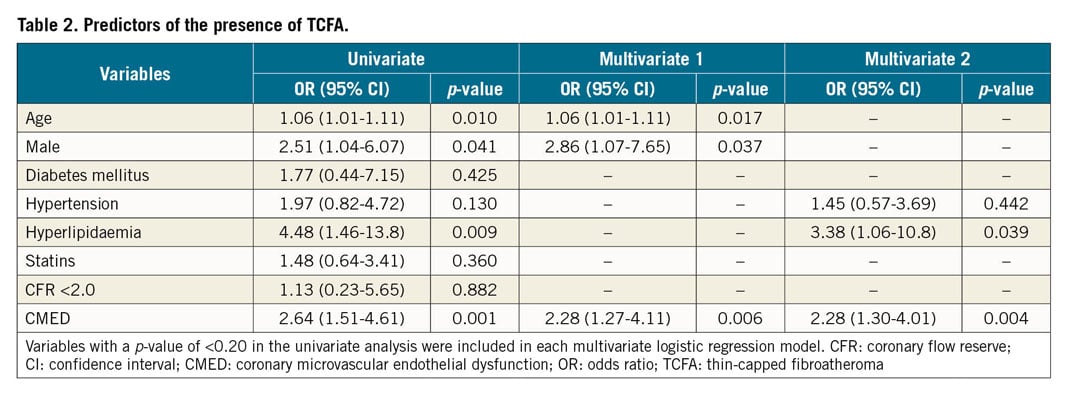
Bonferroni’s correction was used for multiple comparisons. Multivariate logistic regression analysis was performed to identify independent risk factors associated with the presence of TCFA. Potential variables based on the literature13 with p<0.20 in the univariate analysis were included in multivariate logistic regression models. Statistical analyses were performed using SPSS Statistics, Version 25 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA, USA). A two-tailed p-value of <0.05 was considered statistically significant.
Results
PATIENT CHARACTERISTICS
Between July 2005 and February 2018, a total of 190 patients, who were referred for evaluation of chest pain and found to have angiographically normal coronary arteries or non-obstructive CAD (<40% stenosis), underwent comprehensive invasive coronary endothelial function testing and VH-IVUS examination of the LAD. Forty-two patients were excluded because of the predefined reasons listed in Supplementary Figure 1. Of the remaining 148 patients, 87 (59%) met the criteria for CMED. As shown in Table 1, patients with CMED were slightly older and had a higher proportion of men as compared to those without. Other clinical characteristics were comparable between the two groups, including coronary risk factors, type of chest pain, medications, laboratory data, and left ventricular ejection fraction. In both groups, more than 60% were women, less than 10% had diabetes mellitus, approximately 60% had hyperlipidaemia, approximately 40% were obese, and a little less than 50% were on statin therapy. The plasma levels of fasting blood glucose, lipid profile, and a systemic inflammatory marker, high-sensitivity C-reactive protein, were unremarkable and comparable between the two groups.
COMPREHENSIVE INVASIVE CORONARY PHYSIOLOGY STUDY
As summarised in Supplementary Table 1 and Supplementary Figure 2, patients with CMED showed abnormal endothelial function at both microvascular and epicardial levels. Endothelium-dependent CBF increases in response to ACh were evident in a dose-dependent manner in patients with normal coronary microvascular endothelial function, while those were completely abolished in patients with CMED (Supplementary Figure 2A, Supplementary Figure 2B).
In contrast to these endothelium-dependent coronary vascular responses to ACh, endothelium-independent responses to nitroglycerine at both epicardial and microvascular levels were comparable between the two groups (Supplementary Figure 2A-Supplementary Figure 2D). The percentages of patients with abnormal endothelium-independent CFR (<2.0) were also comparable between the two groups, although the mean CFR was slightly but significantly lower in patients with CMED than in those without (Supplementary Table 1). Notably, most patients with abnormal CFR had CMED (n=7/10 [70.0%]: 3 had mild and 4 had severe CMED), whereas most patients with normal coronary microvascular endothelial function had normal CFR (n=58/61 [95.1%]). In addition, the above-mentioned differences in endothelium-dependent coronary vascular responses were not attributable to differences in basal coronary flow rate or haemodynamic parameters because baseline CBF, mean arterial pressure, and heart rate at each point were all comparable between the two groups (Supplementary Table 1, Supplementary Figure 2E, Supplementary Figure 2F).
GREYSCALE INTRAVASCULAR ULTRASOUND FINDINGS
A total of 10,271 greyscale IVUS frames with corresponding VH-IVUS frames from 148 patients (arteries) were analysed. The mean number of analysed frames per artery and the mean length of analysed arteries were similar between the two groups (Supplementary Table 1). Patients with CMED exhibited larger plaque volume index, plaque burden, and plaque thickness, as compared to those without (Supplementary Table 1). When patients with CMED were further divided into mild or severe groups according to the severity of CMED, a dose-effect relationship was found between coronary microvascular endothelial function and IVUS-derived plaque characteristics; plaque burden and plaque volume index increased in accordance with the severity of CMED (Figure 1A, Figure 1B). Change in CBF in response to a maximum dose of ACh showed a significant negative correlation with plaque burden (r=−0.301, p<0.001) and plaque volume index (r=–0.287, p<0.001) (Figure 1C, Figure 1D).
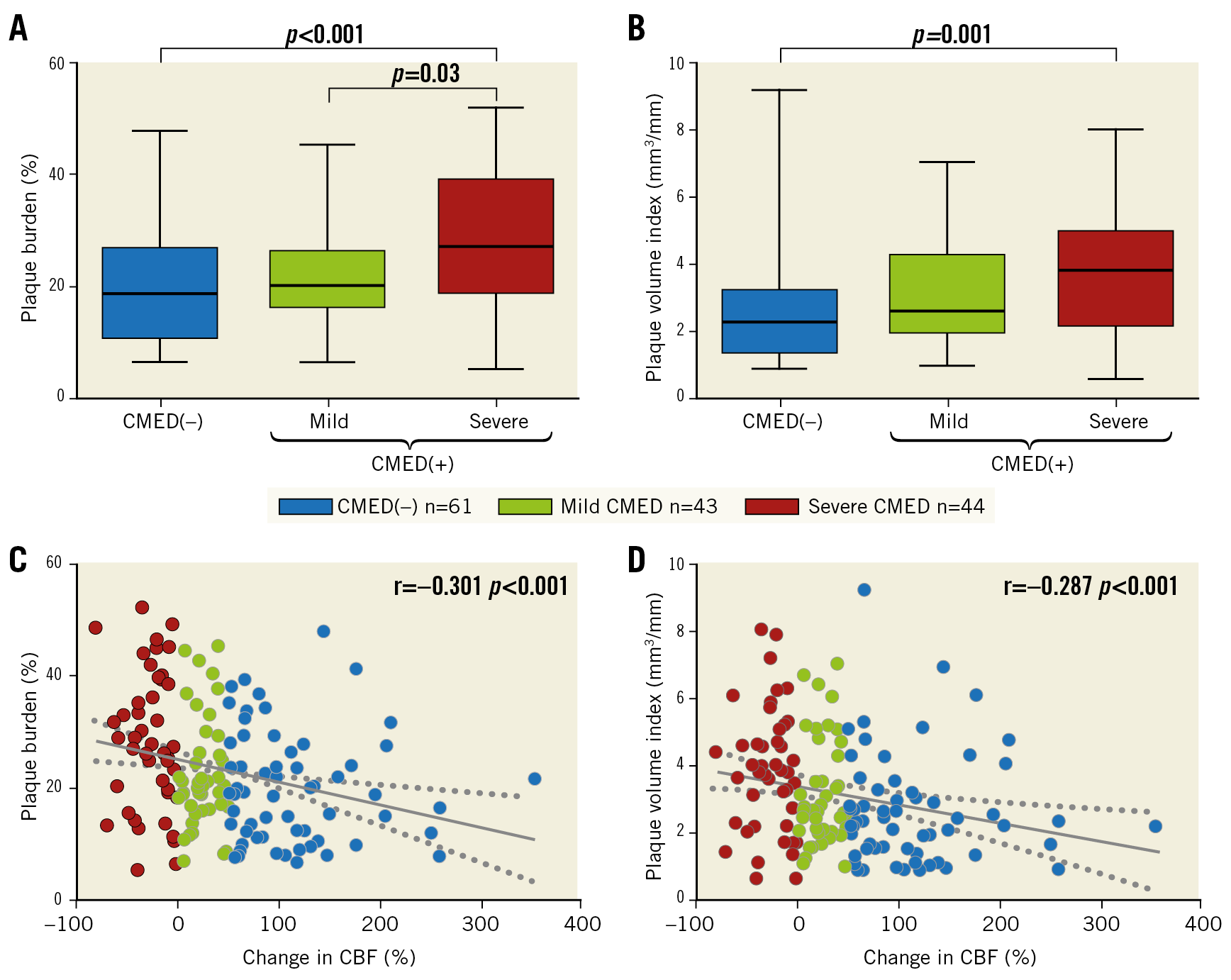
Figure 1. Association of coronary microvascular endothelial function with plaque burden and plaque volume. A) & B) Greyscale IVUS-derived plaque burden and plaque volume index in box and whisker plots. C) & D) Correlations between change in CBF in response to a maximal dose of acetylcholine and plaque burden and plaque volume index. Dotted lines indicate 95% confidence intervals. CBF: coronary blood flow; CMED: coronary microvascular endothelial dysfunction
VIRTUAL-HISTOLOGY INTRAVASCULAR ULTRASOUND FINDINGS
Representative images of VH-IVUS in the absence or presence of CMED are presented in Figure 2A and Figure 2B. Although both patients had angiographically silent atherosclerotic lesions with similar plaque burden and lumen area, they showed distinct differences in the plaque composition, as illustrated. Patients with CMED, as compared to those without, exhibited higher fibrous and necrotic core volume indices in accordance with the severity of CMED (Supplementary Table 1, Figure 3A, Figure 3B). Moreover, patients with CMED had a higher frequency of imaged arteries containing at least one VH-IVUS-derived TCFA in a dose-effect dependent manner (Supplementary Table 1, Figure 3C). In contrast, no association was found between endothelium-independent CFR and coronary plaque characteristics (Supplementary Figure 3).
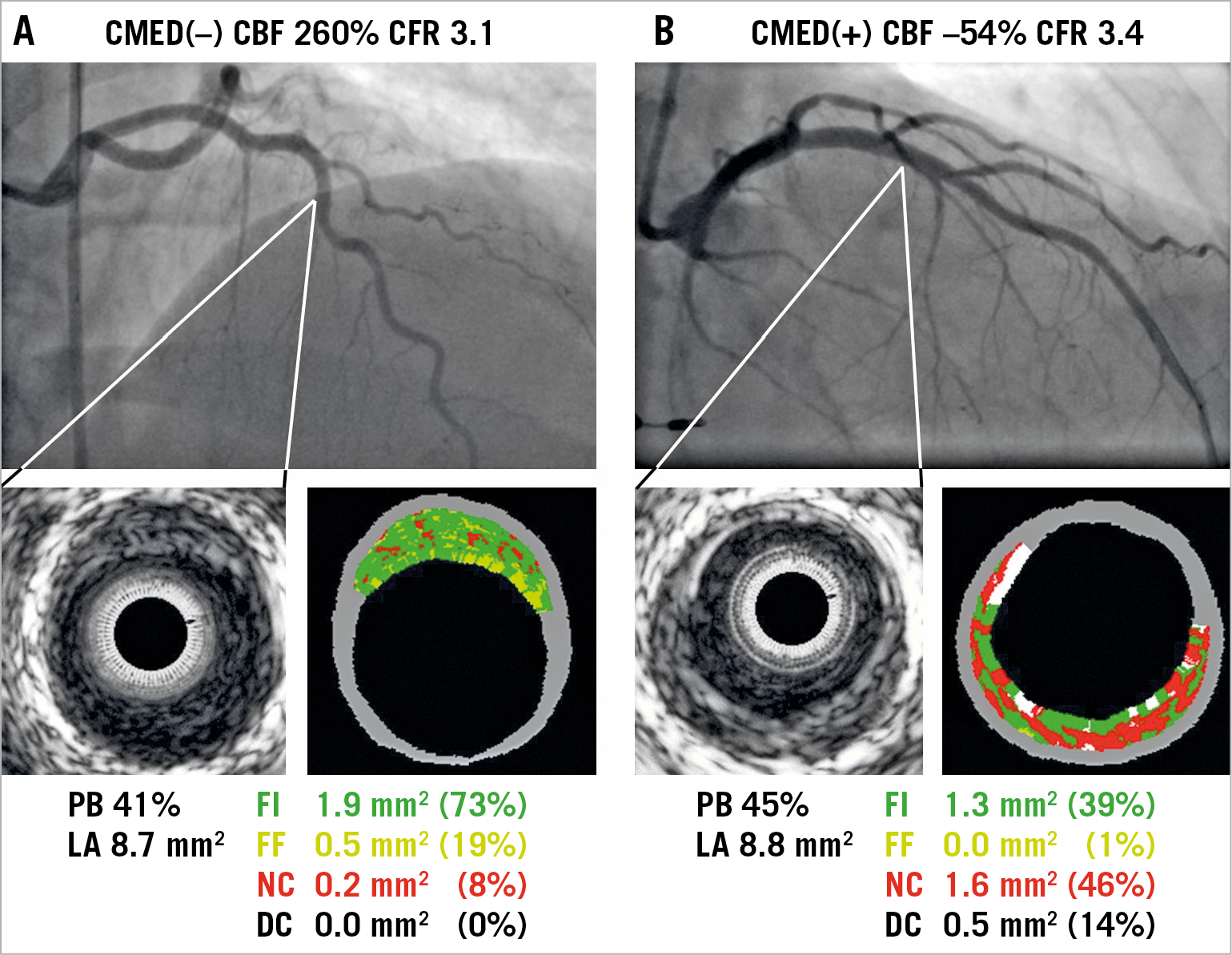
Figure 2. Representative images of coronary angiography, greyscale and VH-IVUS in patients with or without CMED. A) A 53-year-old woman with chest pain showed no significant epicardial coronary artery stenosis and normal coronary microvascular endothelial function. VH-IVUS revealed an angiographically silent fibrous plaque. B) A 59-year-old woman with chest pain showed severe CMED in the absence of significant epicardial coronary artery stenosis and in association with VH-IVUS-derived thin-capped fibroatheroma. CBF: coronary blood flow; CFR: coronary flow reserve; CMED: coronary microvascular endothelial dysfunction; DC: dense calcium; FF: fibro-fatty; FI: fibrous; LA: lumen area; NC: necrotic core; PB: plaque burden
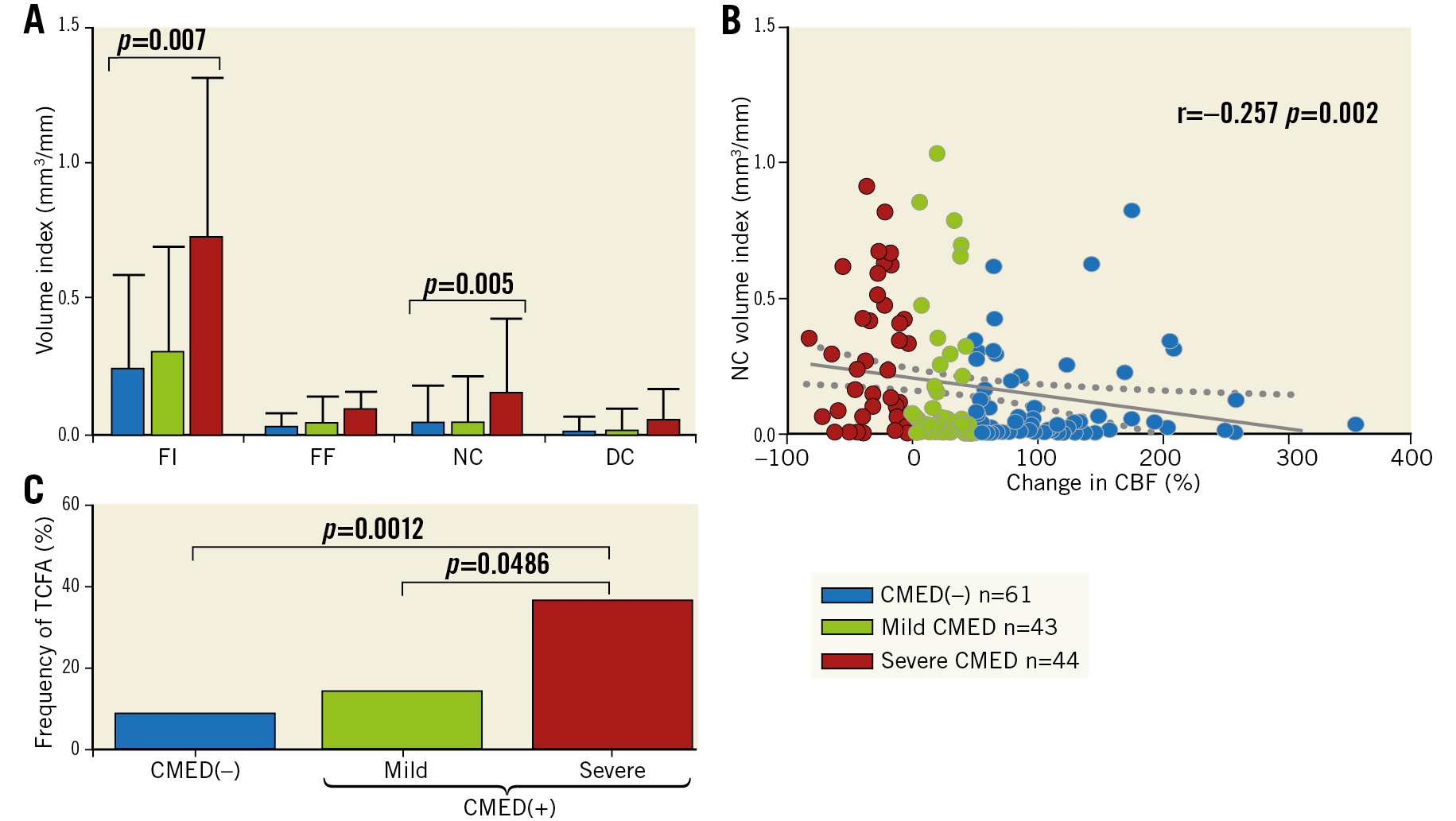
Figure 3. Association of coronary microvascular endothelial function with plaque composition and vulnerability. A) VH-IVUS-derived tissue composition. Values are expressed as median with IQR. B) A correlation between change in CBF in response to a maximum dose of acetylcholine and VH-IVUS-derived NC volume index. C) Frequency of imaged arteries containing at least one VH-IVUS-derived TCFA. CBF: coronary blood flow; CMED: coronary microvascular endothelial dysfunction; DC: dense calcium; FF: fibro-fatty; FI: fibrous; NC: necrotic core
We performed additional analyses restricted to the coronary segments without epicardial coronary endothelial dysfunction in order to examine whether CMED was associated with more advanced plaque characteristics, independently of the presence of epicardial coronary endothelial dysfunction. A total of 409 segments from 107 patients (arteries) without epicardial coronary endothelial dysfunction were analysed (Supplementary Figure 4A). As shown in Supplementary Figure 4B-Supplementary Figure 4D, analysed epicardial segments of LAD coronaries with CMED showed greater plaque burden with more vulnerable characteristics even in the absence of epicardial coronary endothelial dysfunction.
MULTIVARIATE LOGISTIC REGRESSION ANALYSIS
Finally, we performed a multivariate logistic regression analysis to identify predictors of the presence of VH-IVUS-derived TCFA. Among potential variables that might affect plaque vulnerability, CMED was an independent predictor of the presence of VH-IVUS-derived TCFA (Table 2).
Discussion
The novel findings of the present study were twofold. First, CMED was associated with more advanced plaque characteristics in patients with chest pain and non-obstructive CAD even in the absence of epicardial coronary endothelial dysfunction. Second, CMED was an independent predictor of the presence of VH-IVUS-derived TCFA, which is characteristic of rupture-prone vulnerable plaques. These results indicate a potential role of CMED in the progression of coronary atherosclerosis in the early stage of the disease.
Patients with CMED, whether accompanied by epicardial coronary endothelial dysfunction or not, showed more vulnerable plaque characteristics as compared to those without. We have previously demonstrated associations of segmental epicardial coronary endothelial dysfunction with early structural changes in the local vascular wall in patients with non-obstructive CAD using multimodality imaging. Local coronary segments with epicardial endothelial dysfunction showed more advanced characteristics and accelerated progression of the coronary plaques as compared to those without2,3. Such anatomical links at the epicardial level can account for the development and progression of the site-specific coronary plaques in the early stage of the disease. However, the role of CMED in this context was not fully investigated. One possible mechanism for the association of CMED with advanced plaque characteristics can be derived from our recent observation that CMED is associated with low endothelial shear stress in the epicardial coronary artery11. Steady laminar or pulsatile shear stress provides atheroprotective effects on the vascular wall and, conversely, altered oscillatory or low shear stress with disturbed flow promotes atherogenesis through endothelial and vascular smooth muscle cell proliferation, inflammation, lipoprotein uptake, and leukocyte adhesion. Indeed, altered shear stress on the coronary artery wall, whether high or low, has been implicated in the local progression of atherosclerotic coronary plaque20. In the present study, a mechanistic role was, at least in part, attributable to the microcirculation because the same coronary segments with downstream CMED showed more advanced plaque characteristics even in the absence of epicardial coronary endothelial dysfunction (Supplementary Figure 4). Taken together, these observations may provide insight into the underlying mechanisms by which CMED, probably in association with altered patterns of endothelial shear stress, contributes to the development of epicardial coronary atherosclerosis, even though these focal lesions are located upstream to the microcirculation.
The second significant observation of this study was that CMED was an independent predictor of VH-IVUS-derived TCFA in patients with chest pain and non-obstructive CAD. The patients in both groups showed an angiographically similar degree of CAD; however, they showed distinct differences in the angiographically silent plaque characteristics. Patients with CMED exhibited a greater volume of necrotic core and a higher incidence of VH-IVUS-derived TCFA in a dose-effect dependent manner. VH-IVUS-derived TCFA emerged as a surrogate for plaque vulnerability; its clinical importance as a precursor of future coronary events has been repeatedly reproduced16,21. Moreover, this study builds on a recent report showing that coronary microvascular dysfunction is independently associated with a higher prevalence of optical coherence tomography-defined TCFA in patients with advanced coronary lesions, albeit a different method (index of microcirculatory resistance) was used to evaluate coronary microvascular function22. Unlike our study, an endothelium-independent CFR value of <2.0, as evaluated by the same invasive method as our study, was shown to be an independent predictor of the presence of VH-IVUS-derived TCFA in a prior study13. This study did not assess endothelium-dependent coronary microvascular function and included patients with more advanced stages of CAD including acute coronary syndrome with resultant differences in the predictive value of CFR for VH-IVUS-derived TCFA. Considering the possibility that CMED might precede epicardial coronary endothelial dysfunction as well as endothelium-independent coronary microvascular dysfunction in the early phase of CAD11, CMED may enable us to capture patients at risk for future coronary events at an earlier stage of the disease than does endothelium-independent coronary microvascular dysfunction.
CLINICAL IMPLICATIONS: PRIMARY CORONARY MICROCIRCULATORY DYSFUNCTION
This study supports the novel concepts of “primary coronary microcirculatory dysfunction”23 and the “vulnerable patient”24 and provides important implications for practice and research. Patients with chest pain but without angiographical abnormalities are often underdiagnosed and offered no specific treatment or follow-up under the umbrella of “normal” coronary arteries. Contrary to this otherwise common practice, patients with CMED may be at opposite poles; they may be predisposed to the development of more vulnerable coronary atherosclerosis and thus likely destined for future coronary events7,8. Identifying patients at risk for future coronary events could provide significant benefit if they have CMED and may provide physicians with useful information for decision making and risk stratification beyond conventional coronary risk factors. It may be speculated that the development of novel therapies to improve CMED will attenuate the progression of coronary atherosclerosis and that patients with CMED may benefit from early aggressive medical management aimed at improving endothelial function and atherosclerosis upon detection of CMED. To this end, a comprehensive invasive assessment of coronary physiology has been shown to be feasible and of diagnostic value4,6,9. Further research is needed to address how to modulate CMED to improve clinical outcomes of patients with this condition and whether decision making driven by CMED benefits them.
Study limitations
Several limitations should be mentioned in this study. First, although the sample size was relatively large and derived from the prospective database, the results obtained from this retrospective, single-centre, cross-sectional study should be regarded as hypothesis-generating rather than definite evidence. Further research is warranted, especially to establish detailed pathophysiological mechanisms and the cause-effect relationship between CMED and vulnerable plaque characteristics. Second, some of the patient characteristics in the present study were not typical for patients with CAD in general; more than 60% were women and fewer than 10% had diabetes mellitus. Although these characteristics were compatible with those of prior studies that enrolled similar patients with chest pain and non-obstructive CAD4,25,26, a generalisation of the present findings to a wider range of patients with CAD awaits further investigation. Third, ischaemia-inducing testing was not routinely performed in the study patients. However, we have previously demonstrated that contemporary non-invasive stress tests have limited diagnostic accuracy for detecting coronary microvascular dysfunction in patients with chest pain and non-obstructive CAD9,27. Fourth, a previous study suggested the usefulness of the combined use of VH-IVUS and optical coherence tomography to detect TCFA accurately in vivo28. The gold standard for assessment of plaque composition is true histology, which obviously cannot be performed in vivo. However, multiple studies have demonstrated the high predictive accuracy of VH-IVUS in classifying coronary plaque components and phenotypes, including TCFA18,29. Moreover, a large clinical trial has linked VH-IVUS-defined plaque characteristics to adverse clinical outcomes16.
Conclusions
This study highlighted that, independently of conventional coronary risk factors and epicardial coronary endothelial dysfunction, CMED was associated with vulnerable plaque characteristics in patients with early coronary atherosclerosis.
|
Impact on daily practice This study shows that CMED is not uncommon and associated with more advanced plaque characteristics in patients with non-obstructive CAD. CMED is an independent predictor of the presence of VH-IVUS-derived TCFA, which is characteristic of rupture-prone vulnerable plaques. A comprehensive invasive assessment of coronary endothelial function is feasible and of diagnostic value to detect patients with CMED. |
Acknowledgements
We appreciate the effort of the members of the Mayo Clinic Cardiac Catheterization Laboratory and especially the registered cardiovascular invasive specialist Jonella M. Tilford for her excellent technical assistance. Appreciation is also extended to the MSD Life Science Foundation, Public Interest Incorporated Foundation scholarship, Japan.
Funding
This work was supported by the Mayo Foundation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.