
Abstract
Aims: Myocardial bridging (MB), characterised by the epicardial coronary vessel diving into the myocardium, is present in up to one third of adults and is associated with angina and acute coronary syndromes. MB is accompanied by altered blood flow mechanics and regional changes in wall sheer stress. The purpose of this study was to determine the association between myocardial bridging and coronary endothelial dysfunction.
Methods and results: Patients presenting with chest pain and found to have non-obstructive CAD (stenosis <40%) on angiography underwent an invasive assessment of epicardial and microvascular endothelial function. Epicardial endothelial function was assessed by measuring the percent change in coronary artery diameter in response to intracoronary infusions of acetylcholine (%ΔCADAch). Epicardial endothelial dysfunction was defined as a %ΔCADAch of <−20%. Microvascular endothelial function was assessed by the percent change in coronary blood flow in response to intracoronary infusions of acetylcholine (%ΔCBFAch), and microvascular endothelial dysfunction was defined as a %ΔCBFAch of <50%. MB was diagnosed angiographically by identifying the characteristic reduction in minimal luminal diameter during systole. Patients were divided into those with and those without MB, and the frequency of epicardial endothelial dysfunction and microvascular endothelial dysfunction was compared between patients with versus those without MB. Between 1993 and 2012, 1,469 patients (mean age 50.4 years, 35% male) underwent coronary angiography and invasive testing of endothelial function. Two hundred and eight (14.2%) patients were found to have MB in the LAD. Patients with any MB had a significantly higher frequency of endothelial dysfunction within the mid and/or distal vessel segment compared to patients without MB (60.1% vs 50.4%, p=0.012). In multivariate analyses, mid and/or distal vessel MB was a significant predictor of mid and/or distal vessel epicardial endothelial dysfunction (OR 1.44, 95% CI: 1.04-2.00, p=0.029) and of microvascular endothelial dysfunction (OR 1.34, 95% CI: 1.00-1.82, p=0.050).
Conclusions: MB co-localises with epicardial endothelial dysfunction and is significantly associated with microvascular endothelial dysfunction in symptomatic patients with non-obstructive CAD, supporting its potential role as a mechanism for angina in symptomatic patients with MB.
Introduction
Myocardial bridging (MB), characterised by an epicardial coronary artery “diving” into the myocardium and systolic compression of the tunnelled segment, is present on average in one third of adults. However, this number may underestimate the true prevalence due to underdiagnosis1,2. Concurrent pharmacologic provocation testing may help to uncover MB in up to 40% of individuals by enhancing systolic myocardial compression3. MB is generally considered to be a benign condition; however, its presence has been linked to angina, acute coronary syndromes4,5, and left ventricular dysfunction6. Although ischaemia related to MB is thought to explain these clinical manifestations7, systolic compression of the tunnelled segment alone is unlikely to be sufficient to account for severe ischaemia and associated symptoms2,8. We have previously shown that MB is associated with impaired endothelium-dependent epicardial vasorelaxation9, which may exacerbate the myocardial ischaemia beyond structural compression alone, and may play a key role in further understanding the pathophysiology of MB.
Endothelial dysfunction represents one of the earliest stages of atherosclerosis, and is associated with plaque progression and a several fold increased risk of ischaemic cardiac events10,11. In clinical practice it is most often recognised by an abnormal response to endothelium-dependent vasodilating agents such as acetylcholine12. In the coronary vasculature, an attenuated increase or decrease in coronary blood flow (CBF)13,14 in response to acetylcholine marks the presence of microvascular endothelial dysfunction, whereas epicardial vasoconstriction indicates epicardial endothelial dysfunction12.
MB has been shown to lead to regional alterations in concentrations of vasoactive agents such as nitric oxide synthase and endothelin-115 as well as local variations in shear stress and endothelial cell morphology2,15. Endothelial dysfunction is characterised by decreased bioavailability of nitric oxide16, and is also influenced by flow-related shear stress17. However, the link between MB and endothelial dysfunction is incompletely understood. We aimed to evaluate the association of MB and invasively determined epicardial and microvascular coronary endothelial dysfunction in a large cohort of patients presenting with chest pain in the absence of obstructive coronary artery disease (CAD) on angiography.
Methods
STUDY PROTOCOL
The following protocol was approved by the Mayo Clinic Institutional Review Board. Patients were referred by their physician for assessment of chest pain. Consecutive patients presented to the cardiac catheterisation laboratory in the fasting state and vasoactive cardiovascular medications such as nitrates and calcium channel blockers were discontinued for at least 48 hours prior to catheterisation. Routine clinically indicated diagnostic coronary angiography was performed on all patients using standard clinical protocols. Angiograms were reviewed prior to the infusion of any pharmacological agents. Patients with greater than 40% diameter stenosis of any coronary artery were excluded14,18, and the remaining patients underwent an invasive assessment of coronary endothelial function.
After intravenous administration of 5,000-7,000 U of heparin, a Doppler guidewire (FloWire®; Volcano Corp., San Diego, CA, USA) 0.014 inches in diameter within a 3 Fr Slip-Cath® Infusion Catheter (Cook Medical, Bloomington, IN, USA) was positioned into the left anterior descending coronary artery (LAD), 2-3 mm distal to the tip of the infusion catheter. When measuring changes in coronary blood flow to assess microvascular endothelial function, the infusion catheter was always placed in the mid portion of the LAD. Epicardial endothelial function was evaluated by measuring changes in coronary artery diameter separately in each of the proximal, mid, and distal vessel segments. In each case, the infusion catheter with the Doppler guidewire 2-3 mm distal to the tip of the infusion catheter was placed in the segment that was being interrogated, such that the tip of the Doppler guidewire sat approximately in the middle of each respective segment. Coronary artery diameter was measured in the middle of each vessel segment, at the location of the tip of the Doppler guidewire. Additional methodology related to the invasive assessment of coronary endothelial function has been described elsewhere14,18.
We retrospectively reviewed the coronary angiogram reports that were documented at the time of each coronary angiogram for all patients who underwent an invasive assessment of coronary endothelial function. The presence of MB was determined visually at coronary angiography by identifying segments of the coronary artery that underwent the characteristic “milking effect” between systole and diastole8. Bridging was also characterised depending on the segment of the coronary artery in which it was identified (Figure 1).
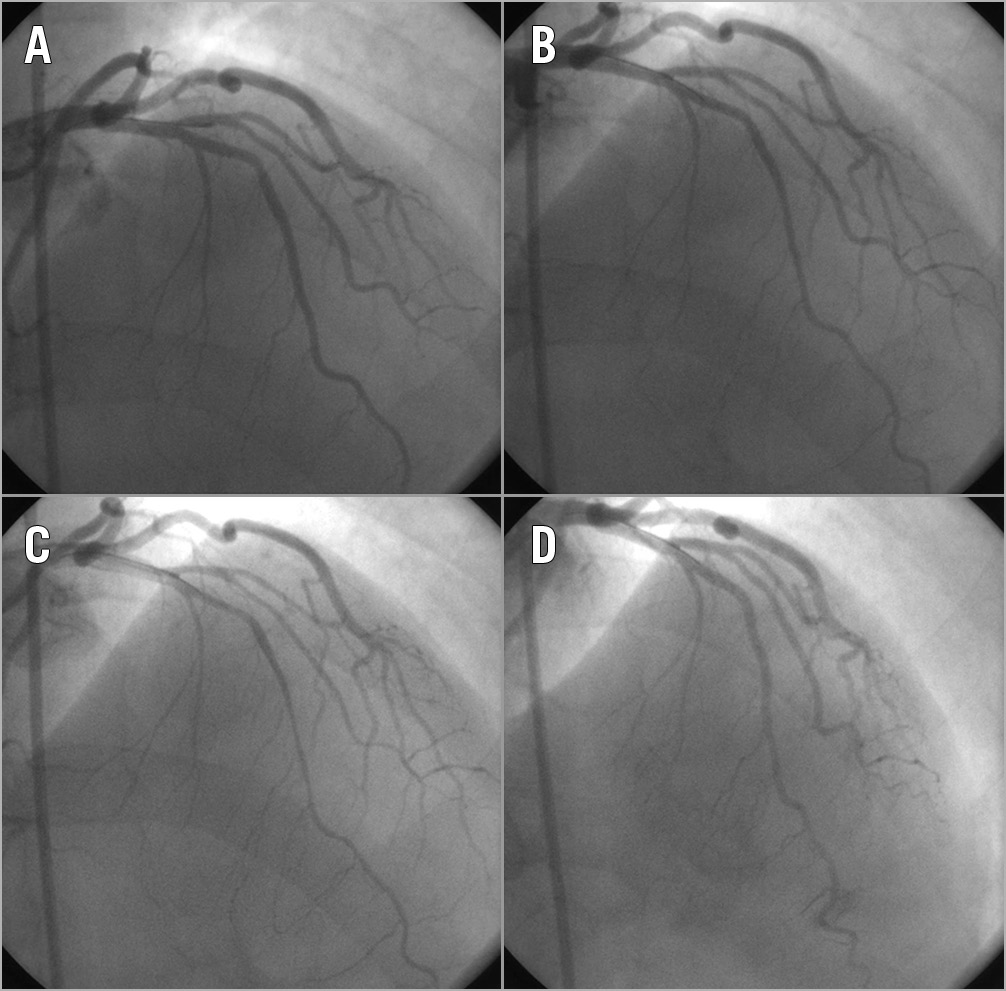
Figure 1. Coronary angiogram images demonstrating mid and distal myocardial bridging in the left anterior descending coronary artery. A) Baseline. B) Bridging in the mid and distal vessel evident with systolic phasic compression. C) After intracoronary infusion of acetylcholine exacerbating vasoconstriction. D) After intracoronary infusion of nitroglycerine resulting in generalised vasodilation helping to uncover mid-distal bridging.
BASELINE CHARACTERISTICS
Data were collected on conventional cardiovascular risk factors including hypertension, diabetes mellitus, hyperlipidaemia, smoking status, body mass index (BMI), and biochemical parameters including serum total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides and creatinine. Smoking was categorised as a history of current smoking, former smoking or never smoking. Hypertension was defined as a history of hypertension treated with antihypertensives; diabetes was defined as a history of diabetes treated with oral medication or insulin, and hyperlipidaemia was defined as a history of total cholesterol levels of >240 mg/dL or treatment with lipid-lowering therapy. All blood levels documented had been drawn within six weeks of the index procedure. Information was also collected on past medical history including a history of myocardial infarction (MI), and other vascular diseases (defined as a documented history of peripheral vascular disease, stroke or transient ischaemic attack).
STATISTICAL ANALYSIS
Patients were stratified by the presence or absence of MB. Continuous variables are presented as a mean (standard deviation) where data are approximately normally distributed and as a median (quartile 1, quartile 3) for skewed data. Categorical variables are presented as frequencies and percentages. Differences between the groups were tested using the Student’s t-test and Wilcoxon rank-sum test for continuous variables and Pearson’s chi-squared test for proportions. Univariate and multivariate logistic regression models were fitted to assess the association between an MB and epicardial endothelial dysfunction and microvascular endothelial dysfunction. All patients with MB had mid and/or distal vessel segment bridging and thus, when assessing the relationship between bridging and epicardial endothelial dysfunction, we identified the percentage of patients with mid and/or distal segment epicardial endothelial dysfunction only and excluded the frequency of endothelial dysfunction in the proximal vessel segment. A p-value less than 0.05 was considered significant, and all statistical analyses were performed using JMP 9 software (SAS Institute, Inc., Cary, NC, USA).
Results
SAMPLE OVERVIEW
Between 1993 and 2012, 1,469 patients (mean age 50.4 years, 35% male) underwent coronary angiography and invasive testing of endothelial function. Two hundred and eight patients were found to have MB (14.2%) on coronary angiography, all within the LAD. Of these patients, 110 (52.9%) had MB in only the mid segment of the vessel, 52 (25.0%) had MB in only the distal and 46 (22.1%) had bridging in both the mid and distal segments of the vessel. No patient had bridging in the proximal segment of the vessel.
Amongst all patients, 151 (10.3%) did not undergo an assessment of epicardial endothelial dysfunction and one patient (0.1%) did not undergo an assessment of microvascular endothelial dysfunction. Six hundred and sixty-two patients (50.2%) had epicardial endothelial dysfunction in at least one segment of their epicardial artery, defined as an abnormal percent change in coronary artery diameter in response to intracoronary infusions of acetylcholine (%ΔCADAch; constriction of vessel diameter of 20% or more in response to intracoronary infusion of acetylcholine), and 763 (51.9%) had microvascular endothelial dysfunction defined as an abnormal percent change in coronary blood flow in response to intracoronary infusion of acetylcholine (%ΔCBFAch; an increase of coronary blood flow of less than 50% after intracoronary infusion of acetylcholine compared to baseline). Of the 662 patients with epicardial endothelial dysfunction, 208 (31.4%) had concomitant microvascular endothelial dysfunction, and 180 patients (27.2%) had isolated epicardial endothelial dysfunction in the absence of microvascular endothelial dysfunction. Overall, 943 patients (64.2%) had evidence of epicardial and/or microvascular endothelial dysfunction. Amongst those with epicardial endothelial dysfunction, 18 patients (4.6%) had isolated proximal vessel endothelial dysfunction, 134 patients (34.5%) had isolated mid vessel endothelial dysfunction, 236 patients (60.8%) had isolated distal vessel endothelial dysfunction, 14 (3.6%) had proximal and mid vessel endothelial dysfunction, 221 (57.0%) had mid and distal vessel endothelial dysfunction, and 39 patients (10.1%) had proximal, mid and distal vessel endothelial dysfunction. Amongst patients with epicardial endothelial dysfunction, 591 patients (89.3%) had mid and/or distal vessel endothelial dysfunction, and no proximal endothelial dysfunction. This group included all patients with mid and distal vessel endothelial dysfunction, isolated mid vessel endothelial dysfunction and isolated distal vessel endothelial dysfunction (Figure 2). There were no procedure-related complications such as coronary dissection, myocardial infarction, stroke, life-threatening arrhythmia, major bleeding or death.
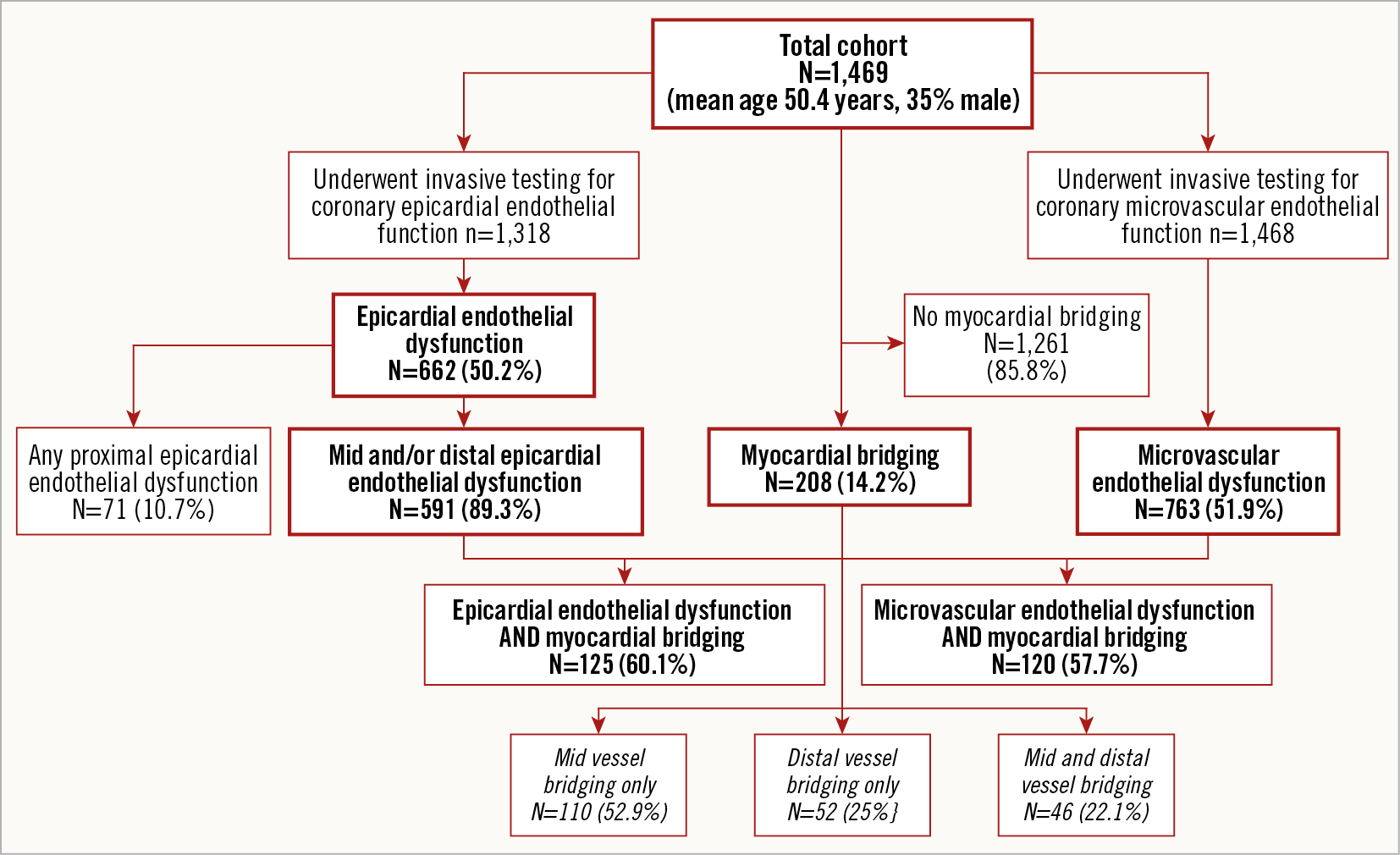
Figure 2. Flow chart outlining the number of patients who underwent an invasive assessment of coronary endothelial function and the proportions of patients with epicardial and microvascular endothelial dysfunction and myocardial bridging.
PATIENT CHARACTERISTICS
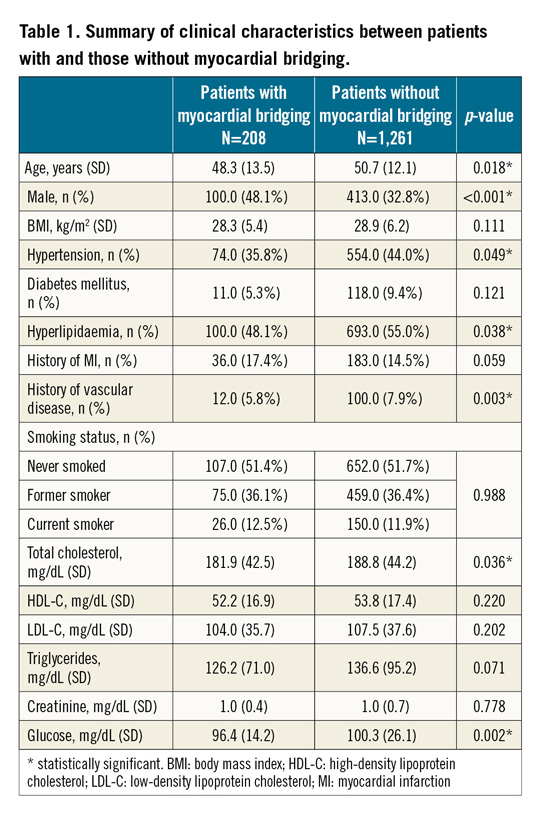
Table 1 outlines differences in clinical profile between patients with versus those without MB. Overall, patients with MB were younger, and there was a higher proportion of males compared to those without MB. In addition, patients with MB had a lower frequency of hypertension, hyperlipidaemia, and history of vascular disease compared to patients without MB; they also had a significantly lower total cholesterol, and glucose. All other clinical and biochemical parameters were not significantly different between the groups.
FREQUENCY OF ENDOTHELIAL DYSFUNCTION IN PATIENTS WITH MYOCARDIAL BRIDGING
When assessing the prevalence of epicardial endothelial dysfunction amongst patients with versus those without MB, 1,318 patients underwent an invasive assessment of epicardial endothelial function. Patients who did not undergo a concurrent invasive assessment of epicardial endothelial dysfunction were not significantly different compared to those who did undergo an assessment for endothelial dysfunction with regard to demographic and clinical variables.
Patients with any MB, localised to the mid and/or distal vessel segment, had a significantly higher frequency of mid and/or distal endothelial dysfunction (60.1% vs 50.4%, p=0.012) compared to patients without any MB (Figure 3). The frequency of proximal vessel endothelial dysfunction did not differ significantly between patients with versus those without any MB (8.2% vs 6.8%, p=0.488). Patients with any MB had a tendency towards a higher frequency of microvascular endothelial dysfunction compared to patients without any MB; however, this did not reach statistical significance (57.7% vs 51.0%, p=0.075) (Figure 3). When stratifying all patients by age, patients aged ≤50 years with any MB had a significantly higher frequency of microvascular endothelial dysfunction compared to patients without any MB (57.3% vs 44.2%, p=0.010), suggesting that age may modify the effect of MB on the frequency of microvascular endothelial dysfunction. Amongst patients aged >50 years, the frequency of microvascular endothelial dysfunction did not vary significantly between patients with versus those without MB.
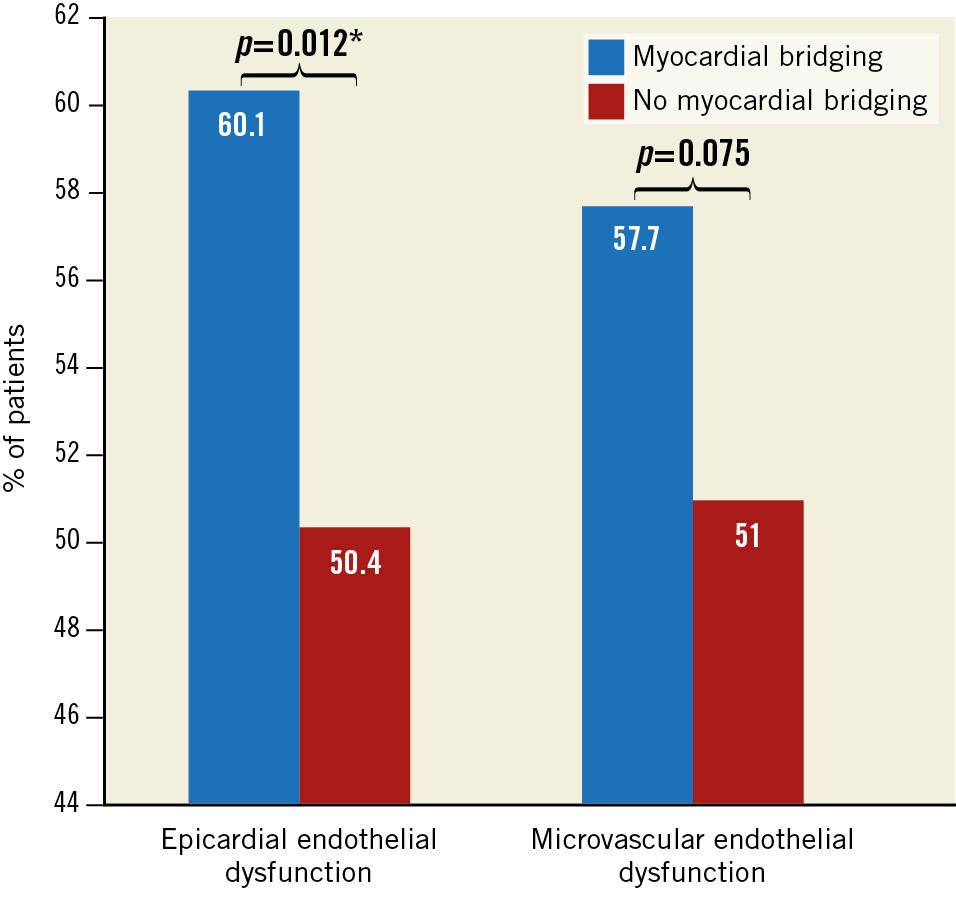
Figure 3. Bar chart comparing differences in percentage of patients with mid and/or distal vessel epicardial endothelial dysfunction and microvascular endothelial dysfunction between patients with and without mid and/or distal vessel myocardial bridging. Amongst 1,469 patients in the cohort, 151 (10.3%) did not undergo an assessment of epicardial endothelial dysfunction and one patient (0.1%) did not undergo an assessment of microvascular endothelial dysfunction. The frequency of myocardial bridging versus no myocardial bridging in patients with epicardial endothelial dysfunction (n=1,318) and for those with microvascular endothelial dysfunction (n=1,468) is shown. * statistically significant.
UNIVARIATE AND MULTIVARIATE ASSOCIATION
In a univariate analysis, MB in the mid and/or distal segment was significantly associated with mid and/or distal vessel epicardial endothelial dysfunction, odds ratio (OR) 1.50, 95% confidence interval (CI): 1.09-2.06, p=0.012. This association remained significant after adjusting for age, sex, smoking status, hypertension, hyperlipidaemia, diabetes mellitus, and vascular disease (Supplementary Table 1). MB in the mid and/or distal segment was not significantly associated with proximal vessel endothelial dysfunction in a univariate or multivariate analysis.
After stratifying by age, there was a significant association between mid and/or distal MB and microvascular endothelial dysfunction amongst patients aged ≤50 years, OR 1.69, 95% CI: 1.13-2.52, p=0.010, but not amongst patients aged >50 years. In a multivariate analysis, MB was associated with borderline significance with microvascular endothelial dysfunction (Supplementary Table 2).
Discussion
The current study has four main findings. First, MB is angiographically present in 14.2% of patients presenting with chest pain and non-obstructive CAD. Second, patients with MB are younger, more likely to be male, and have fewer traditional cardiovascular risk factors compared to those without MB. Third, in patients with MB, epicardial endothelial dysfunction often co-localises with the same segment of the vessel, suggesting an underlying association between the two pathophysiologic processes. Fourth, MB was significantly associated with microvascular endothelial dysfunction. Thus, the current study supports a potential role for MB as a mechanism of endothelial dysfunction in patients with chest pain and non-obstructive CAD.
The current study demonstrated that MB is prevalent in patients presenting with chest pain and non-obstructive CAD at angiography and that the majority of bridging occurred in the mid segment of the vessel. Our findings are in keeping with the results of other studies which have shown a prevalence of MB ranging from 0.5% to 29.5%19,20,21,22. Importantly, the current study evaluated 1,469 patients, making it, to our knowledge, the largest study to investigate the prevalence of MB in patients with angina and non-obstructive CAD. The current study also extends the findings of those previous studies by evaluating the clinical profile of patients with MB. We showed that patients with MB were on average younger and more likely to be male than patients without MB. Patients with MB also had a lower prevalence of hypertension, hyperlipidaemia and a history of vascular disease, as well as statistically significant, though modestly lower, levels of total cholesterol and glucose, suggesting that conventional cardiovascular risk factors are less likely to contribute to endothelial dysfunction in patients with MB.
We previously showed that MB is associated with impaired endothelium-dependent epicardial vasorelaxation in a smaller cohort9, suggesting that epicardial endothelial dysfunction may contribute to lumen obstruction. The current study extends these findings by evaluating a large unselected population of patients with chest pain who underwent routine coronary angiography, including evaluation of MB, as well as invasive assessment of coronary endothelial dysfunction. We found that MB had a higher frequency of epicardial endothelial dysfunction within the same segment of the vessel that was affected by the bridge compared to patients without MB. MB was a significant predictor of epicardial endothelial dysfunction in the same bridged segment both in a univariate analysis as well as after adjusting for conventional cardiovascular risk factors. Other studies have also linked MB and endothelial dysfunction within the bridged segment9,23. We further extend the findings of these previous studies by the spatial selectivity of this link, because we found no difference in the frequency of epicardial endothelial dysfunction proximal to the segment with bridging between patients with versus those without MB. Furthermore, a novel aspect of the current study is the relationship with the coronary microcirculation. We found that MB is a significant predictor of microvascular endothelial dysfunction after adjusting for conventional cardiovascular risk factors, further supporting the role of endothelial dysfunction as the potential mechanism of myocardial ischaemia in symptomatic patients with MB. These findings may also have implications on therapy in patients with MB.
Despite being viewed as a benign condition, MB has been linked to angina and acute coronary syndromes4,5. MB-related ischaemia is thought to underpin the symptoms and cardiovascular events experienced by these patients. Indeed, studies have shown reversible perfusion abnormalities during stress myocardial single photon emission computed tomography in patients with MB24. However, the degree of phasic vessel compression is unlikely to account for severe ischaemia and the associated clinical manifestations in patients with MB8. Thus, other contributing factors may play a role. Alterations in blood flow haemodynamics within the MB segment are consistent with a high pressure and high shear stress chamber25. High intravascular pressure has been shown to be associated with impaired endothelium-mediated relaxation in animal models26. We recently showed that flow-related shear stress is an important factor for endothelial dysfunction in patients with early stages of coronary atherosclerosis27, as extremes in shear stress towards either end of the spectrum can be harmful to the health and function of the endothelium17,28. The results of the current study demonstrate that epicardial endothelial dysfunction is significantly associated with MB and co-localises with the bridged segment of the involved vessel. Endothelial dysfunction represents the first stage of atherosclerosis, and may lead to ischaemia as well as an increased risk of cardiovascular events10,11. Thus, it could play a role in explaining the syndrome of chest pain and adverse cardiac events experienced by patients with MB.
Previous studies have suggested that the segment immediately proximal to the bridge in patients with MB is most prone to atherosclerosis development29. This is thought to be related to relatively lower flow rates at the entrance of the bridge with concomitant low and oscillatory shear stress2, increased vascular cell adhesion molecule-1 expression30 and a pro-atherogenic endothelial cell phenotype31. However, in the current study we did not find a significantly higher frequency of endothelial dysfunction in the segment proximal to the bridge in patients with as compared to those without MB. The precise relationship between MB, regional flow haemodynamics, shear stress and endothelial dysfunction remains poorly understood and is evidently complex and multifaceted. For example, levels of nitric oxide and endothelin-1 have been shown to be lower in bridged segments compared to segments proximal and distal to the bridge15. Discordant variations in either chemical could shift the balance to pathologic vasoconstriction and differences in regional endothelial function depending on the location of the bridge. Indeed, while some reports have shown that bridged segments are typically spared from atherosclerosis32, other reports have shown atherosclerotic disease in bridged segments33. Further work is required to delineate better the precise relationship between MB, the resultant changes in haemodynamic flow and atherogenesis.
The current study also shows that MB was significantly associated with microvascular endothelial dysfunction measured as an abnormal coronary blood flow response to acetylcholine. It may be that a variety of vasoactive chemicals such as nitric oxide synthase, and endothelin-1 whose concentrations become altered in patients with MB15 influence microvascular function remotely, particularly as endothelial dysfunction is itself characterised by altered bioavailability of vasoactive chemicals16. Interestingly, age appeared to modify the effect of MB on microvascular endothelial dysfunction. The bioavailability of different vasoactive substances is known to vary with age34; thus, there may be interdependence between bridging and patient age which in turn leads to variations in vasoactive substance bioavailability and subsequent endothelial dysfunction. This relationship requires further study.
Limitations
This study has a number of limitations. First, this study evaluated patients referred to a tertiary referral centre for coronary angiography and so represents a select population. Second, we did not follow patients prospectively for cardiovascular events nor was the direct relationship between MB, endothelial dysfunction and ischaemia demonstrated. Third, our analyses did not factor in the length or depth of each bridged segment, which may play a role in whether patients have endothelial dysfunction and to what degree.
Conclusions
MB co-localises with epicardial endothelial dysfunction and is significantly associated with both epicardial and microvascular endothelial dysfunction in patients presenting with chest pain and non-obstructive CAD, supporting its potential role as a mechanism for angina in symptomatic patients with MB. These findings also shed new light on the potential pathophysiological basis for the link between MB and cardiovascular events which may be mediated through endothelial dysfunction.
|
Impact on daily practice The current study shows that myocardial bridging is angiographically present in 14.2% of patients presenting with chest pain and non-obstructive coronary disease. These patients are younger, more likely to be male, and have fewer traditional cardiovascular risk factors compared to patients without bridging. In patients with myocardial bridging, epicardial endothelial dysfunction often co-localises within the same segment of the vessel and is independently associated with both epicardial and microvascular endothelial dysfunction. These findings suggest an underlying association between the two pathophysiologic processes, support the potential role of endothelial dysfunction as a mechanism for angina in symptomatic patients with myocardial bridging, and could shed new light on the potential pathophysiological basis for the link between myocardial bridging and cardiovascular events which may be mediated through endothelial dysfunction. |
Funding
This work was supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750) and the Mayo Foundation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

