Abstract
Increasing evidence has shown that coronary spasm and vasomotor dysfunction may be the underlying cause in more than half of myocardial infarctions with non-obstructive coronary arteries (MINOCA) as well as an important cause of chronic chest pain in the outpatient setting. We review the contemporary understanding of coronary spasm and related vasomotor dysfunction of the coronary arteries, the pathophysiology and prognosis, and current and emerging approaches to diagnosis and evidence-based treatment.
Coronary spasm (CS) is defined as diffuse or focal severe vasoconstriction of the coronary arteries resulting in impaired myocardial perfusion1. It was first described in 1959 by Prinzmetal as anginal pain associated with transient ST-segment elevations or depressions on an electrocardiogram (ECG)23. CS was historically thought to be a condition predominantly affecting middle-aged males in East Asia and was rare in European and North American populations; more recent data suggest that it may be a common cause of myocardial infarction with non-obstructive coronary arteries (MINOCA) in European and North American patients4, with a more equal sex distribution5. Microvascular spasm has been recognised in the last 20 years and is more poorly characterised. CS, both epicardial and microvascular, remains an important and likely underdiagnosed cause of chronic chest pain in the outpatient setting. We describe advances in our understanding and the definition of CS, techniques for diagnosis, epidemiology and the natural history of the condition, and therapeutic modalities.
Coronary spasm and vasomotor dysfunction
Initially, CS was considered a disease of the epicardial coronary arteries, but, as understanding increased, spasm of the arterioles within the microcirculation was also identified. CS is best considered as part of a spectrum of vasomotor disorders affecting the coronary arteries and may coexist with coronary microvascular dysfunction (CMD). Patients with vasomotor disorders have dysregulation of vasoconstriction and vasodilatation of the coronary vasculature, leading to episodic ischaemia and chest pain. Patients may present with MINOCA or sudden cardiac death1 or, more commonly, with chronic chest pain – either angina with non-obstructive coronary arteries (ANOCA) or ischaemia with non-obstructive coronary arteries (INOCA).
Pathophysiology
The coronary arteries are dynamic regulators of coronary flow; they constrict and dilate to regulate myocardial perfusion and match supply to demand. Vasodilation occurs due to an increase in endothelium-derived relaxing factors including nitric oxide (NO) and relaxation of the vascular smooth muscle cells (VSMCs). The principal abnormality in CS is hyperreactivity of the VSMCs within the media, with increased sensitivity to intracellular calcium concentrations that results in an increased propensity towards hypercontraction6. The endothelium and the perivascular adipose tissue (PVAT) within the adventitia6 both secrete signalling factors that modulate vasoconstriction and vasodilation (Figure 1). In epicardial coronary arteries, in vitro studies have shown induction of spasm regardless of whether the endothelium was intact or removed, suggesting that hypercontraction of the VSMCs is the major abnormality7.
The final pathway for CS is Rho-kinase (RoK) inhibition of myosin light chain phosphatase, resulting in inhibited myosin dephosphorylation and prolonged actin-myosin coupling6. RoK activity is increased in patients with CS, especially in those with active anginal symptoms8. Inflammation of the PVAT may lead to upregulation of RoK activity and VSMC hyperreactivity6. The association between PVAT inflammation and CS may explain why CS is more prevalent in smokers since nicotine is associated with chronic low-grade inflammation.
Microvascular spasm was described in 2002 in a cohort of patients with known epicardial CS. Patients with ischaemic ECG changes, chest pain and increased coronary sinus lactate in response to lower doses of acetylcholine (ACh) in the absence of severe epicardial spasm were considered to have microvascular spasm, and this occurred in 25% of patients in this cohort with known epicardial CS9. Uncertainty exists regarding the overlap of microvascular CS and endothelium-dependent CMD. Both are diagnosed via chest pain, ECG changes and ischaemia in response to ACh10. However, microvascular spasm is considered an abnormality of the microvascular VSMC, while endothelium-dependent CMD results from an imbalance in endothelium-derived vasoconstricting and vasodilating agents. In a normal heart, low-dose ACh causes microvascular vasodilation and a >50% increase in coronary blood flow (CBF) due to an increase in endothelium-derived vasodilating factors. At higher doses, patients susceptible to CS will manifest VSMC-mediated coronary vasoconstriction. However, VSMC-mediated vasoconstriction may also occur at lower doses of ACh in some patients with epicardial CS. Therefore, it is not possible to reliably distinguish isolated microvascular spasm from endothelium-dependent CMD in vivo (Figure 2).
The guidelines also reflect this uncertainty. The European Association of Percutaneous Cardiovascular Interventions guidelines label patients with chest pain/ECG changes but no significant epicardial vasospasm in response to ACh as experiencing microvascular spasm, whereas the American College of Cardiology guidelines label this event endothelium-dependent CMD11. Ischaemia due to endothelium-dependent CMD is associated with increased circulating endothelium-derived vasoconstricting agents and decreased cyclic guanosine monophosphate12, and CBF improves with L-arginine, a signalling pathway mediated by the endothelium10 (Figure 1). Therefore, endothelium-dependent CMD and microvascular CS are not different names for the same condition. Doppler CBF measurements demonstrate that approximately 30% of patients have a decrease in CBF with ACh, while the remainder have an increase13. We consider patients with a decrease in CBF to have microvascular spasm, since CBF will decrease if the microcirculatory resistance arterioles vasoconstrict, while a CBF increase of 0-49% is diagnosed as endothelium-dependent CMD.
Patients may have both epicardial and microvascular CS. Diagnosis is via ACh rechallenge. If epicardial spasm is provoked with ACh, nitroglycerine (e.g., 200 μg) is given to reverse epicardial vasoconstriction. ACh is then redosed at 100-200 μg, and if symptoms/ECG changes reoccur, the patient has coexisting microvascular spasm. Microvascular spasm is less responsive to nitroglycerine, as there is diminished biotransformation of nitroglycerine in vessels <100 μg in diameter14.
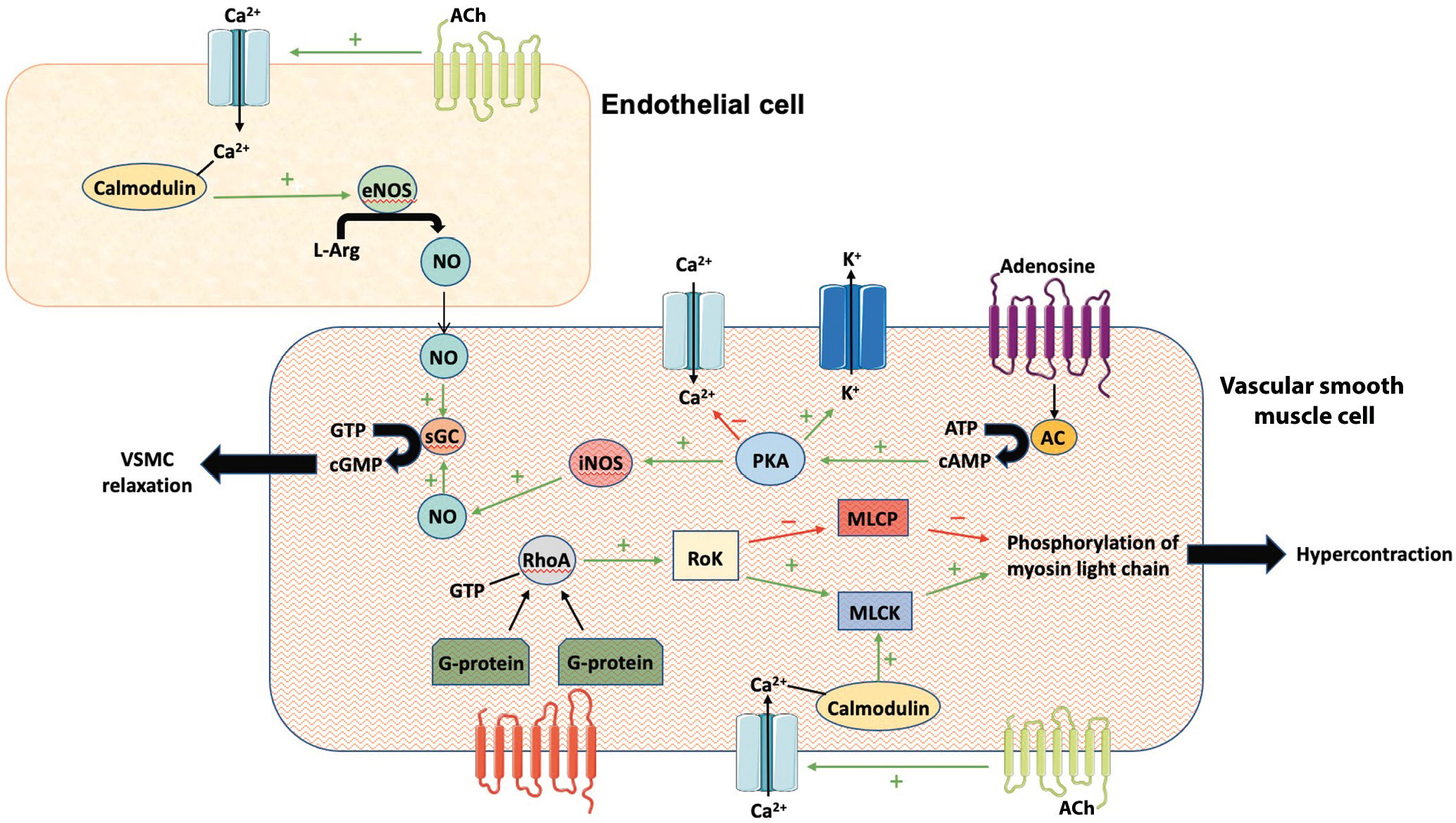
Figure 1. Cellular interplay between endothelial- and VSMC-mediated signalling pathways that promote relaxation and contraction. AC: adenylyl cyclase; ACh: acetylcholine; ATP: adenosine triphosphate; CA2+: calcium; cAMP: cyclic adenosine monophosphate; cGMP: cyclic guanosine monophosphate; eNOS: endothelial nitric oxide synthase; GTP: guanosine triphosphate; iNOS: inducible nitric oxide synthase; L-Arg: L-arginine; K+: potassium; MLCK: myosin light chain kinase; MLCP: myosin light chain phosphate; NO: nitric oxide; PKA: protein kinase A; RhoA: Ras homolog family member A; RoK: Rho-kinase; sGC: soluble guanylate cyclase; VSMC: vascular smooth muscle cell
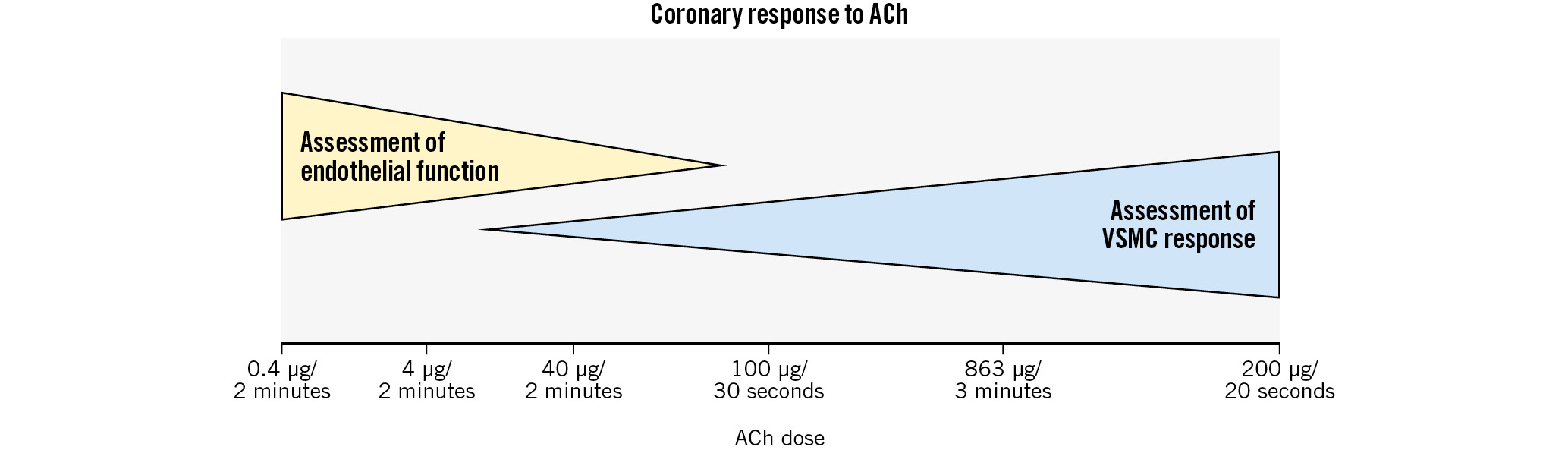
Figure 2. Acetylcholine (ACh) allows diagnosis of endothelium-dependent CMD and CS. Endothelial function is assessed at lower doses of ACh, while patients with CS have VSMC-mediated vasoconstriction at higher doses of ACh. The overlap between the two doses makes it difficult to reliably differentiate endothelium-dependent CMD from isolated microvascular spasm. The x-axis includes common dosing patterns used in different protocols worldwide. CMD: coronary microvascular dysfunction; CS: coronary spasm; VSMC: vascular smooth muscle cell
Epidemiology and prevalence of CS
The estimated prevalence of CS varies widely, between 4-49% in large case series4151617 (Table 1), likely reflecting differences in patient selection (particularly ethnicity and presence/absence of CAD), inconsistent inclusion of microvascular CS, and differing provocation agents and diagnostic criteria for CS. The true incidence in INOCA and MINOCA patients remains unknown, since provocation testing is not routinely performed and prior studies have been in highly selected patient populations. Additionally, most studies of MINOCA did not involve routine provocation testing, which likely contributed to the underestimation of the true prevalence of CS. Women are less likely to have typical angina or undergo angiography, and underdiagnosis may be more common in women18. CS frequently coexists with CAD, and early studies of “variant angina” were in patients with severe epicardial fixed stenoses with superimposed CS causing ST-elevation1920212223. As understanding improved, patients with the same clinical presentation but mild/no underlying epicardial CAD were identified2425262728.
Several small series in patients with MINOCA estimate CS prevalence to be between 16% and 71%416172930 (Table 1). In series which included both microvascular and epicardial CS, 50-70% of spasm was epicardial, and the remaining spasm was microvascular, defined as ECG changes/chest pain in response to ACh in the absence of epicardial vasospasm − a definition which likely also captured patients with endothelium-dependent CMD. Estimates for CS prevalence in patients with ANOCA (Table 1) appear markedly higher in Asian populations, particularly in Japan153132. A small comparative study demonstrated higher rates of impaired vasoreactivity in Japanese compared to European patients with CAD (80% vs 37%) 7-10 days after MI33; however, no studies have elucidated whether this relates to genetic or environmental factors. Epicardial CS appears to be more common in men and microvascular CS in women34, with the overall prevalence of CS in patients with MINOCA being similar between sexes4.
CS commonly coexists with myocardial bridging (MB). In one study, 60% of patients with MB, defined as >30% phasic compression on angiography, had significant CS (>70% vasoconstriction) within the bridged segment during ACh infusion (Figure 3)35. Patients with MB and CS were more likely to have recurrent angina and hospitalisation for chest pain compared to patients with MB without CS35. Atherosclerosis is more likely to form proximal to a myocardial bridge due to non-laminar flow in this area, while the increased mechanical and shear stress secondary to compression within the bridge are thought to lead to endothelial dysfunction and increased susceptibility to CS36.
Table 1. Provocative studies of CS, divided by severity of coronary artery disease.
| Study | Population | ACh dose | ER dose | Diagnostic criteria | Results: % positive spasm test | Complications | ||
|---|---|---|---|---|---|---|---|---|
| MINOCA | ||||||||
| Kanazawa et al, 199729 Japan |
20 patients (age 57±10 years, 70% male) with rest angina; | 50 µg then 100 µg | 40 µg | Not defined | 100% | None | ||
| 50% had ST-elevation with chest pain; 60% had no CAD, and the remainder had <75% CAD | ||||||||
| Da Costa et al, 200117 France | 91 patients (age 50±13 years, 63% male) with MINOCA | 40 µg | Narrowing >70% | 16% | None | |||
| Ong et al, 200816Germany | 488 patients (age 66±12 years, 65% male) with ACS. 86/116 with no culprit lesion/alternative diagnosis given ACh | 20-100 µg LCA 80 µg RCA if no spasm in LCA | Narrowing >75% with reproduction of symptoms. If symptoms and ECG changes but no epicardial spasm, diagnosis of microvascular CS. | 49% of patients given ACh | None | |||
| Montone et al, 20184 Italy | 80 consecutive patients (age 63±11 years, 50% male) with MINOCA, no coronary thrombus/unstable plaque | 20-200 µg LCA 20-50 µg RCA | 8-64 µg LCA 8-40 µg RCA | Narrowing >90% with symptoms and ischaemic ECG changes. If symptoms and ECG changes but no epicardial spasm, diagnosis of microvascular CS. | 46%: of these, 65% epicardial spasm, 35% microvascular spasm | 2 transient bradyarrhythmias | ||
| INOCA | ||||||||
| Suzuki et al,199230 Japan | 26 patients (age 54±10, 91% male), 11 with typical vasospastic angina (1 had >50% atheroma) and 15 with chest pain (8 had >50% atheroma) | 25-100 µg | 1-30 µg | Narrowing >99% with angina and ischaemic ECG changes | ACh: 82%, ER: 100% in vasospastic angina group None in CP group | None | ||
| Harding et al, 199315 North America | 3,447 patients (median age 51, IQR 43-56, 55% male) with <50% CAD and with stable (32%), progressive (55%) and unstable (12%) angina | 50-150 µg IV | Narrowing >75% | 4% | 11 patients (0.03%): MI in 4, VT/VF in 7 | |||
| Wang et al, 200232 Taiwan | 93 patients (age 57±14, 59% male) with suspected ischaemia and CAD <50% | 1-30 µg | Narrowing >90% with chest pain and ischaemic ST-elevation changes | 71% in MINOCA (23 patients)30% in INOCA (70 patients) | None | |||
| Sueda et al,200331 Japan | 193 patients (age 62±10 years, 62% male) with <50% CAD | 20-100 µg LCA 20-80 µg RCA | 16-64 µg LCA10-40 µg RCA | Narrowing >99% | ACh: 33% ER: 32% | None | ||
| Predominantly obstructive CAD | ||||||||
| Bertrand et al, 198219France | 1,089 patients undergoing coronary angiography | 400 µg IV | Narrowing >75% | Group A: 1.2% spasm; 89% had normal/near-normal angiograms | 0.6% (4 VF, 1 VT, 1 transient CHB) | |||
| Group A: 248 with atypical chest painGroup B1: 117 with exertional angina | Group B1: 4% spasm; 20% mild CAD | |||||||
| Group B2: 138 with exertional and rest angina | Group B2: 14% spasm; 42% mild CAD | |||||||
| Group C: 203 with rest angina; 78/203 had ST- elevation during angina | Group C: 38% spasm; 46% mild CAD | |||||||
| Group D1: 116 with transmural MI <6 weeks before | Group D1: 20% spasm; 7% minimal CAD | |||||||
| Group D2: 65 with transmural MI >6 weeks before | Group D2: 6% spasm; 5% mild CAD | |||||||
| Group E: 154 with valvular heart disease | Group E: 2% spasm;86% mild CAD | |||||||
| Group F: 49 with cardiomyopathy | Group F: 0% spasm; 100% normal angiograms | |||||||
| Excluded severe left main/3-vessel disease | ||||||||
| Mark et al, 198420 North America | 109 patients with variant angina with transient ST-segment elevation (spontaneous or after ER) | 300 µg IV | Narrowing >75% | 9/12 (75%) | None | |||
| Yasue et al, 198621 Japan | 28 patients (83% male) with chest pain plus ST-elevation; 72% had >50% CAD | 10-80 µg LCA10-50 µg RCA | Narrowing >25% | 93% | Transient bradyarrhythmias with injection of ACh into RCA | |||
| Nosaka et al, 198723 Japan | 3,000 consecutive patients | Total or subtotal occlusion | Group I: 22% | None | ||||
| Group I: 1,145 with angina (653 at rest) | Group II: 23% | |||||||
| Group II: 398 with MI | Group III: 1.2% | |||||||
| Group III: 648 with atypical chest pain | Group IV: 3.7% | |||||||
| Group IV: 809 with non-ischaemic heart disease | Majority of group I/II had obstructive CAD | |||||||
| Sueda et al, 199922 Japan | 685 patients (age 63±8 years, 70% male) | 20-100 µg LCA 20-80 µg RCA | Narrowing >99% with chest pain and/or ECG changes | 32% | 0.3% haemodynamic instability with ACh | |||
| Mixed CAD/INOCA | ||||||||
| Cipriano et al, 197929 | 90 patients (mean age 48 years, 52% male): 18 with typical angina, 56 atypical chest pain, 9 variant angina, 7 heart transplant | 0.05-0.25 mg | Narrowing >85% | 12% | None | |||
| Okumura et al, 198824 | Group 1: 70 patients (mean age 57 years, 85% male) with chest pain plus ST-elevationGroup 2: 93 patients (mean age 54 years, 69% male) with atypical chest pain | 20-100 µg LCA20-50 µg RCA | 200 µg IV | 100% occlusion or severe vasoconstriction plus chest pain and/or ECG changes | Group 1: 90%, 36% with significant CADGroup 2: 1%,12% with significant CAD | Transient bradycardia with ACh into RCA | ||
| Sueda et al, 200025 Japan | 40 patients with rest angina and ischaemia on ECG24 patients with atypical chest painTrial of triple provocation testing: ACh, ER, then ACh + ER | 20-100 µg LCA 20-80 µg RCA | 16-64 µg LCA10-40 µg RCA | Narrowing >99% plus chest pain and/or ECG changes | Rest angina: ACh, 55%; ER, 33%; ACh+ER, 92%Atypical chest pain:ACh, 0%; ER, 0%; ACh+ER, 8% | None | ||
| Sueda et al, 200426 Japan | 1,508 patients undergoing first coronary angiography | 20-100 µg LCA20-80 µg RCA | 16-64 µg LCA10-40 µg RCA | Narrowing >99% | ACh: 36%ER: 30% | 1% VT, 0.3% severe hypotension, 0.1% cardiac tamponade 1 prolonged spasm >15 min with ER testing138 (16%) transient AF with ACh | ||
| Komatsu et al, 201627 | 47 patients (age 43±13 years, 94% male) who survived OOHA with no organic heart disease. Normal LV function | 20-100 µg LCA20-50 µg RCA | Narrowing >90% plus chest pain or ECG changes | CS alone: 15%CS+OOHA: 51% | PAF 10%Transient bradyarrhythmia 16%VA 3% | |||
| ACh: acetylcholine; ACS: acute coronary syndrome; AF: atrial fibrillation; CAD: coronary artery disease; CHB: complete heart block; CS: coronary spasm; ECG: electrocardiogram; ER: ergonovine; INOCA: ischaemia with non-obstructive coronary arteries; IQR: interquartile range; IV: intravenous; LCA: left coronary artery; LV: left ventricular; MI: myocardial infarction; MINOCA: myocardial infarction with non-obstructive coronary arteries; OOHA: out-of-hospital cardiac arrest; PAF: paroxysmal atrial fibrillation; RCA: right coronary artery; VA: ventricular arrhythmia; VF: ventricular fibrillation; VT: ventricular tachycardia | ||||||||
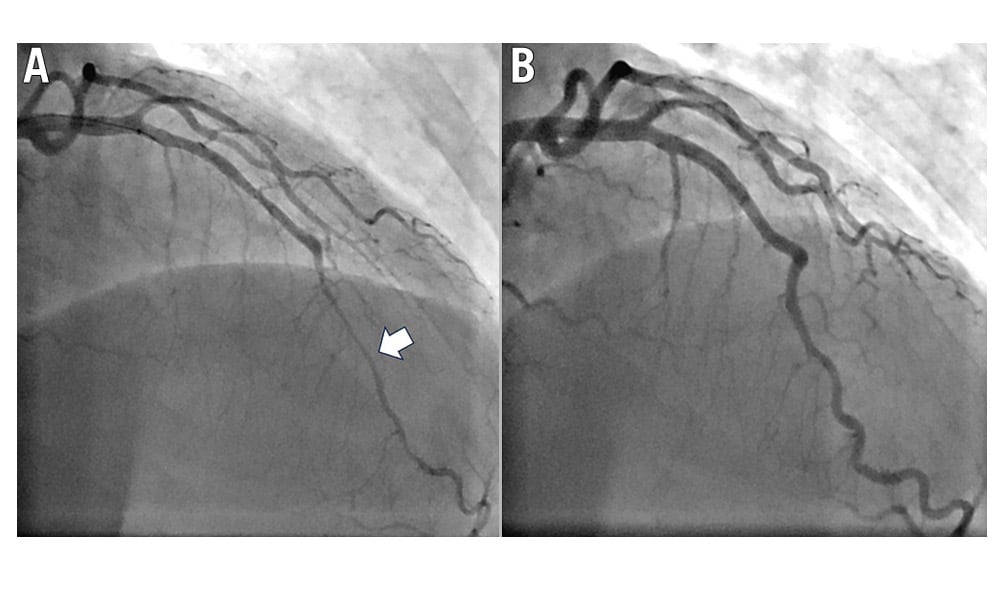
Figure 3. Coronary angiography of the bridged segment of the LAD. A) Coronary spasm is observed during ACh infusion. B) Resolution of spasm following nitroglycerine administration. ACh: acetylcholine; LAD: left anterior descending artery
Invasive provocation testing
The gold standard for diagnosis is invasive provocative testing using ACh or, less commonly, ergonovine (ER) during cardiac catheterisation with continuous monitoring of symptoms and ECG (Figure 4, Figure 5). ACh is short-acting and, therefore, needs to be administered as an intracoronary bolus or infusion. The administration of ER can be intracoronary or intravenous. Calcium channel blockers (CCBs) and long-acting nitrates are usually held for 48 hours and short-acting nitrates for 6 hours prior to testing.
Diagnostic criteria and provocation testing protocols vary between institutions. The Coronary Vasomotor Disorders International Study Group (COVADIS) group proposed a standardised definition to address these limitations: 1) a spontaneous episode of angina that is nitrate responsive and has at least one of the following characteristics: occurs at rest (especially between late night and early morning), marked reduction in exercise tolerance in the morning, precipitated by hyperventilation, or suppressed by CCBs; 2) transient ischaemic ECG changes in at least two contiguous leads; and 3) transient coronary stenosis of >90% with associated angina or ischaemic ECG changes, either spontaneously or in response to a provocative stimulus2. The definition of microvascular spasm often follows the COVADIS criteria of chest pain and ECG changes during provocation testing but, in the absence of severe epicardial vasoconstriction37, with the caveats of overlap with endothelial-dependent CMD as detailed above.
The COVADIS definition has high specificity. However, a major limitation in the diagnosis of CS has been the large variation in practice for provocation testing, particularly with respect to the dose and rate of administration of the provocation agent38. Using only lower-dose ACh (e.g., 50 μg over 3 minutes), the >90% threshold for vasospasm will underdiagnose epicardial CS, while only using higher doses may underdiagnose endothelium-dependent CMD (Figure 2). This emphasises the importance of a standardised diagnostic protocol that includes doses and rate of administration. With regard to the percentage of vasospasm considered diagnostic, studies in MINOCA patients used >70-75% vasoconstriction516 to define coronary spasm, and it is our experience that chest pain and ischaemic ECG changes can be seen at this threshold.
ACh acts on the muscarinic receptors of endothelial cells and VSMCs. In a healthy artery, ACh will stimulate endothelial-derived NO release, resulting in mild vasodilation of the epicardial artery39. In patients with endothelial dysfunction, there is inadequate endothelial release of NO, and ACh injection results in VSMC contraction and epicardial vasoconstriction39 (Figure 1). ACh may be administered via an intracoronary infusion catheter or bolus injections. A common protocol is at 10-20 μg, 50 μg then 100 μg in the left coronary artery over 30 seconds41516171920212223242526272829303132. ER is an alternative, less commonly used provocation agent. ER binds to serotonergic 5HT2 receptors to induce vasoconstriction. Intracoronary ER is administered in 5 to 10 μg increments up to a maximum of 50 μg41516171920212223242526272829303132. Some series found ACh to be more sensitive than ER, particularly in men, with spasm provoked in 81% of males versus 54% with ER40, and it is therefore our preferred agent.
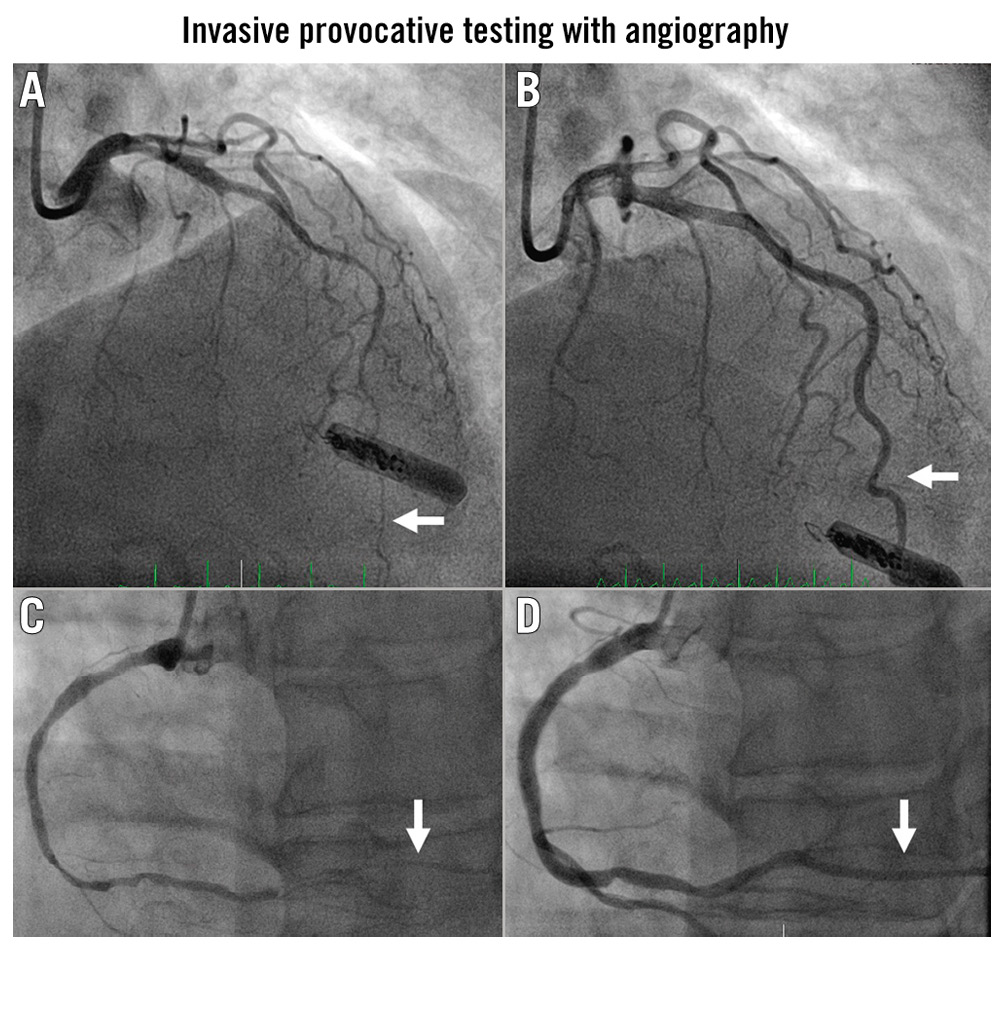
Figure 4. Angiography of the mid- to distal LAD (white arrows). A) After ACh administration and B) after intracoronary nitroglycerine. C) The RCA with obliteration of the PDA associated with inferior ST-elevation and chest pain. D) The same artery after nitroglycerine. ACh: acetylcholine; LAD: left anterior descending artery; PDA: posterior descending artery; RCA: right coronary artery
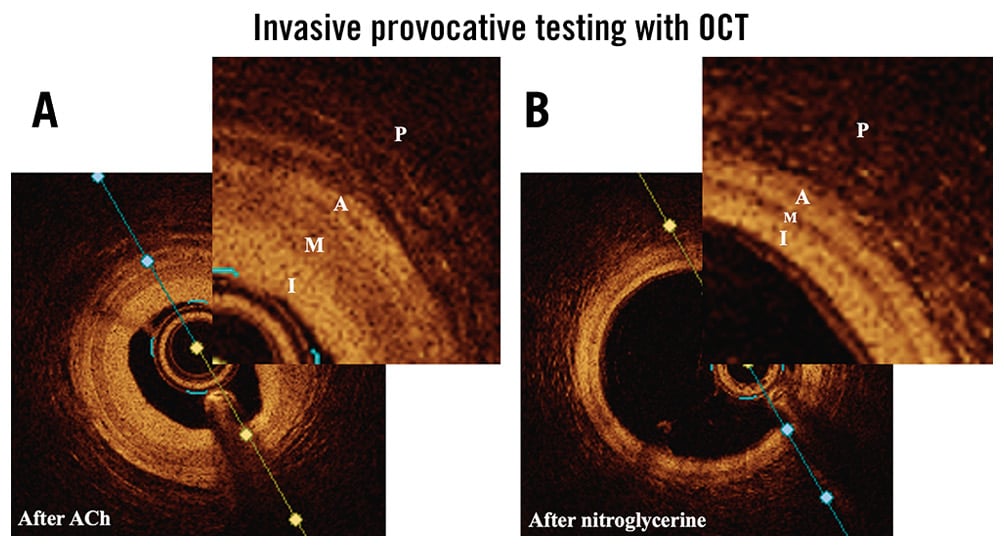
Figure 5. OCT of the same LAD artery. A) After administration of 50 µg ACh and B) after nitroglycerine. Ninety percent spasm was induced with higher doses of ACh but would not have allowed high-quality OCT acquisition. During CS, the VSMC in the media (M) contract and the media is therefore thicker. A: adventitia; ACh: acetylcholine; CS: coronary spasm; I: intima; LAD: left anterior descending; OCT: optical coherence tomography; P: perivascular adventitial tissue; VSMC: vascular smooth muscle cell
Invasive assessment of CMD
Invasive testing for CS is usually performed in conjunction with assessment for CMD. ACh is used to diagnose both CS and endothelium-dependent CMD, while the administration of adenosine allows diagnosis of endothelium-independent CMD. The ratio of CBF at rest and CBF with adenosine is the coronary flow reserve (CFR). CFR >2.5 is normal, while CFR <2.0-2.5 is diagnostic of endothelium-independent CMD. CFR and microvascular resistance may be measured invasively using a Doppler or thermodilution wire41. Endothelium-independent CMD may also be diagnosed non-invasively using positron emission tomography or magnetic resonance imaging, with measurement of myocardial perfusion at rest and during adenosine administration; the CFR can then be calculated from the ratio of these measurements42.
Invasive testing is generally safe, with mild adverse reactions in 9% of cases (mostly transient atrial fibrillation)41 and <0.5% with serious complications (Table 1)39.
Management
In patients with classic symptoms of CS and absence of obstructive CAD, an empirical trial of CCBs can be both diagnostic and therapeutic43. Further testing may be indicated if symptoms persist despite medical therapy or if there is diagnostic uncertainty (Table 2).
A management algorithm is shown in Figure 6. Smoking cessation is key44. The first-line pharmacological treatment is a CCB, which prevents vasoconstriction by inhibiting the flow of calcium into the VSMCs. Overall, 40% of patients will be rendered angina-free with CCBs45. Non-dihydropyridine (DHP) CCBs are the preferred first-line agent43, while a combination of non-DHP and DHP CCBs is recommended for ongoing symptoms. Moderate to high doses are often needed, e.g., verapamil 240-480 mg daily, diltiazem 180-540 mg daily, nifedipine 60-120 mg daily46. CCBs may be more effective if taken at night14.
Short-acting nitrates are first-line agents for acute attacks47. Nitrates increase local concentrations of NO, leading to vasodilation47. Long-acting nitrates should be avoided if possible, since tolerance may occur; however, they remain a third-line agent48. The NO donor and arterial potassium adenosine triphosphate channel agonist nicorandil47, the RoK inhibitor fasudil49, and renin-angiotensin system (RAS) inhibitors50 are all second-line alternatives.
Statins decrease symptoms when used in combination with CCBs, likely by improving endothelial function51, and are recommended in patients with CS and hyperlipidaemia (low-density lipoprotein >100 mg/dl). L-arginine, a precursor to NO, is effective in patients with reduced endothelial NO-synthase activity45.
Treatment of MB with coexisting CS may be challenging. CCBs reduce myocardial contractility and decrease hypercontractility. In patients with a haemodynamically significant bridge and symptoms refractory to medical treatment, unroofing surgery is effective at relieving systolic compression and may also improve endothelial function within the bridged segment52.
The optimal treatment of microvascular spasm remains unclear. CCBs are less effective in CMD than epicardial CS53. In patients with ECG changes/chest pain with ACh, in the absence of epicardial spasm (likely a combination of microvascular spasm and endothelium-dependent CMD), 30% had persistent chest pain despite treatment with CCBs and angiotensin-converting enzyme inhibitors (ACEi)54. Nitrates are less effective in relieving microvascular spasm55, while RAS inhibition may be more effective in microvascular compared to epicardial CS50. In general, we follow the same treatment algorithm as for epicardial CS but with earlier consideration of ACEi.
In patients who have had ventricular arrhythmias or cardiac arrest secondary to CS, recurrent arrhythmias may occur, even with maximally tolerated medical therapy24. Patients who presented with ventricular arrhythmia/aborted sudden cardiac death (SCD) had a poorer prognosis in one study (12% vs 1% with 4-year cardiac mortality)56, but similar in another (3% vs 2%)57, compared to CS alone. The role of implantable cardioverter defibrillators (ICDs), in addition to medical therapy in CS plus aborted SCD, is still debated but often recommended based on these data5658. ICDs are not recommended in CS without documented ventricular arrhythmias/cardiac arrest.
Percutaneous coronary intervention (PCI) is not generally recommended for CS, as spasm is likely to recur outside of the stented segment59. However, in selected patients with focal CS refractory to medical therapy, PCI may be reasonable59. Sympathectomy, involving ablation of the T2-T4 sympathetic ganglia, decreased angina in one small study60; however, there was no angiographic re-evaluation to assess if vasoconstriction was resolved. Less invasive methods such as spinal cord stimulators may be more appropriate.
Patients should avoid ergot-containing and anticholinesterase agents, cocaine and amphetamines. Serotonin-containing medications should only be used with caution.
Table 2. COVADIS indications for invasive provocation testing2.
| Class I – strong indications | |
|---|---|
| History suspicious for CS, including: rest angina relieved by nitrates, diurnal variation in symptoms, angina at rest without obstructive CAD, symptoms unresponsive to empirical CS therapy | |
| ACS presentation with no culprit lesion | |
| Unexplained cardiac arrest | |
| Unexplained syncope with antecedent chest pain | |
| Recurrent angina at rest following successful PCI | |
| Class IIa – good indications | |
| Patients diagnosed with CS non-invasively and unresponsive to therapy | |
| Determine the site and mode of spasm in patients with documented spontaneous CS | |
| Class IIb – controversial indications | |
| Patients diagnosed with CS non-invasively and responsive to therapy | |
| Class III – contraindications | |
| Emergent ACS | |
| Severe fixed multivessel CAD | |
| Severe myocardial dysfunction | |
| No symptoms suggestive of CS | |
| ACS: acute coronary syndrome; CAD: coronary artery disease; COVADIS: Coronary Vasomotor Disorders International Study Group; CS: coronary spasm; PCI: percutaneous coronary intervention | |
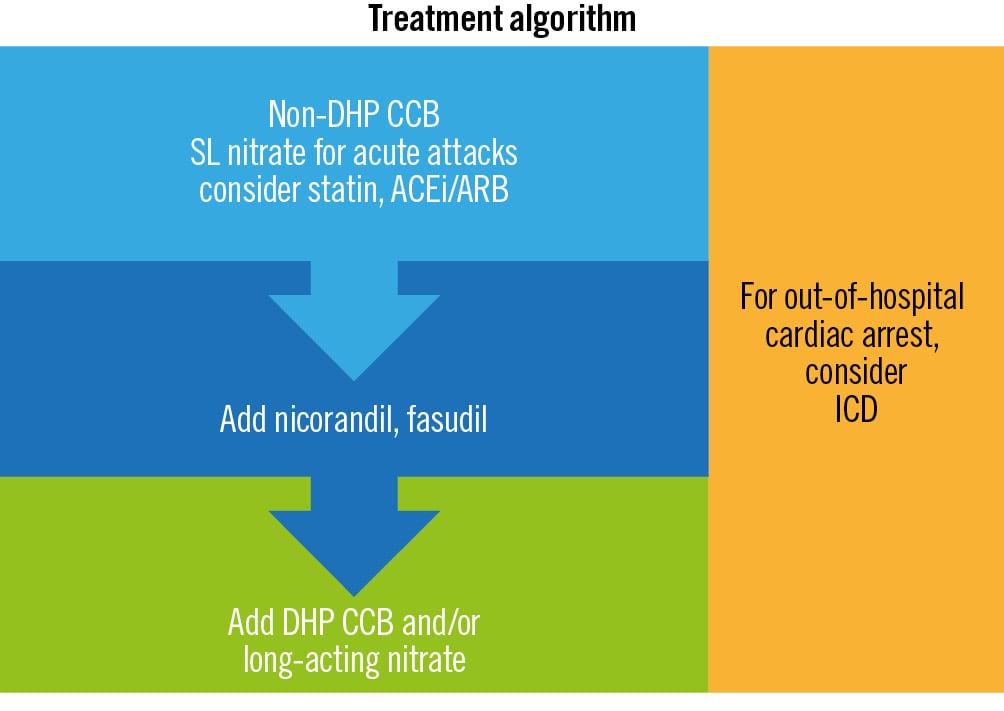
Figure 6. Management algorithm for CS. ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers; CCB: calcium channel blocker; CS: coronary spasm; DHP: dihydropyridine; ICD: implantable cardioverter defibrillator; SL: sublingual
Prognosis
Studies reporting prognosis are heavily dependent on the population, particularly in the presence of concomitant CAD (Table 3, Figure 7)417203256575861626364656667. Prognosis is generally favourable in CS without CAD, acute coronary syndrome or arrhythmia, although recurrent hospitalisation for angina is common. Prognosis was worse with concomitant CAD42061626364656667. Patients with MINOCA tend to have worse prognoses than those with INOCA417203256575861626364656667, particularly if CS is present (major adverse cardiac events rates 13% vs 5%)55.
Spontaneous remission may be seen without medical therapy in 30% of cases68, but long-term CCB maintenance is generally recommended to reduce future risk of arrhythmia or MI (Central illustration).
Table 3. Studies reporting prognosis of CS.
| Study | n | Population | CAD/LV function | Prognosis |
|---|---|---|---|---|
| Waters et al, 198364 Canada | 169 | Hospitalised with variant angina | 63% with >70% CAD; 24% abnormal LV function | 5-year survival: 87%5-year MI-free survival: 75%5-year survival with <70% CAD: 98% |
| Mark et al, 198420 USA | 109 | Variant angina | 92% with >75% CAD; 17% EF <40% | 5-year survival: 76%5-year infarct-free survival: 59% |
| Nakamura et al, 198761 Japan | 349 | Vasospastic angina | 20% with no CAD; 47% fixed stenosis <75%, 33% fixed stenosis >75%; no data on LV function | 2% SCD 5% MI at 3.4-year follow-up 48 patients with no CAD: 0 SCD, 2 MI, 4 VT/VF |
| Walling et al, 198765 Canada | 217 | Variant angina | 60% with >70% CAD; 23% abnormal LV function | 5-year survival: 89% 5-year MI-free survival (total population): 69% 5-year MI-free survival with <70% CAD: 68% |
| Yasue et al, 198862 Japan | 245 | Hospitalised with variant angina | 60% with >75% CAD; 6% abnormal LV function | 5-year survival: 97% 5-year MI-free survival: 83% 5-year survival with <75% stenosis: 82% |
| Da Costa et al, 200117 France | 91 | 91 with MINOCA compared to 91 controls with acute MI plus CAD >50% | 0% with >50% CAD; mean LVEF 60% (47-73%) | 35-month survival: 95% in MINOCA, 92% in MI+CAD. |
| Wang et al, 200232 Taiwan | 93 | Patients presenting to emergency department with suspected cardiac ischaemia and insignificant CAD | No significant CAD; mean LVEF 69% (60-78%) | Median 25-month follow-up. ER positive group: 97% survival, 95% MACE-free. Troponin I-positive group: 96% survival, 91% MACE-free. |
| Ong et al, 201166 Germany | 44 | Suspected ACS with no culprit lesion on angiogram | No significant CAD; median LVEF 70% (62-78%) | 3-year survival: 99%3-year coronary event-free survival: 100% |
| Takagi et al, 201157 Japan | 1,429 | Vasospastic angina | 14% with >50% CAD, 2% OOHA | 5-year survival: 98% 5-year MACE free survival: 91% (MI, unstable angina, HF, appropriate ICD shock) 5-year MI: 0.6% |
| Matsue et al, 201258 Japan | 23 | VA plus vasospastic angina | No documented CAD, normal LV function | Median follow-up 2.9 years: 100% survival, 83% free of VF |
| Cho et al, 201667 South Korea | 986 | Vasospastic angina presenting with (n=149) or without ACS (n=837) | 7% with >50% CAD | 5-year MI: 4% in the non-ACS group, 10% in the ACS group5-year all-cause mortality: 2% in non-ACS, 6% in ACS |
| Ahn et al, 201656 South Korea | 188 | Variant angina with aborted SCD, compared to 1,844 patients with variant angina without aborted SCD | Angiographically normal coronary arteries; no structural heart disease | 5-year cardiac death: 11.5% vs 1.3%5-year rates of VT: 14% |
| Montone et al, 20184 Italy | 37 | Patients with MINOCA: 24/80 epicardial spasm; 13/80 microvascular spasm | 1% obstructive CAD; median LVEF 58% (54-62) | 5-year survival: 62%5-year MI-free survival: 72%5-year cardiac death-free survival: 78% |
| Epicardial CAD is expressed as “x% with >y% CAD” e.g., 63% with >70% CAD means 63% of patients had an epicardial stenosis of >70%. ACS: acute coronary syndrome; CAD: coronary artery disease; CS: coronary spasm; EF: ejection fraction; ER: ergonovine; HF: heart failure; ICD: implantable cardioverter defibrillator; LV: left ventricular; LVEF: left ventricular ejection fraction; MACE: major adverse cardiac events; MI: myocardial infarction; MINOCA: myocardial infarction with non-obstructive coronary arteries; OOHA: out-of-hospital cardiac arrest; SCD: sudden cardiac death; VA: ventricular arrhythmia; VF: ventricular fibrillation; VT: ventricular tachycardia | ||||
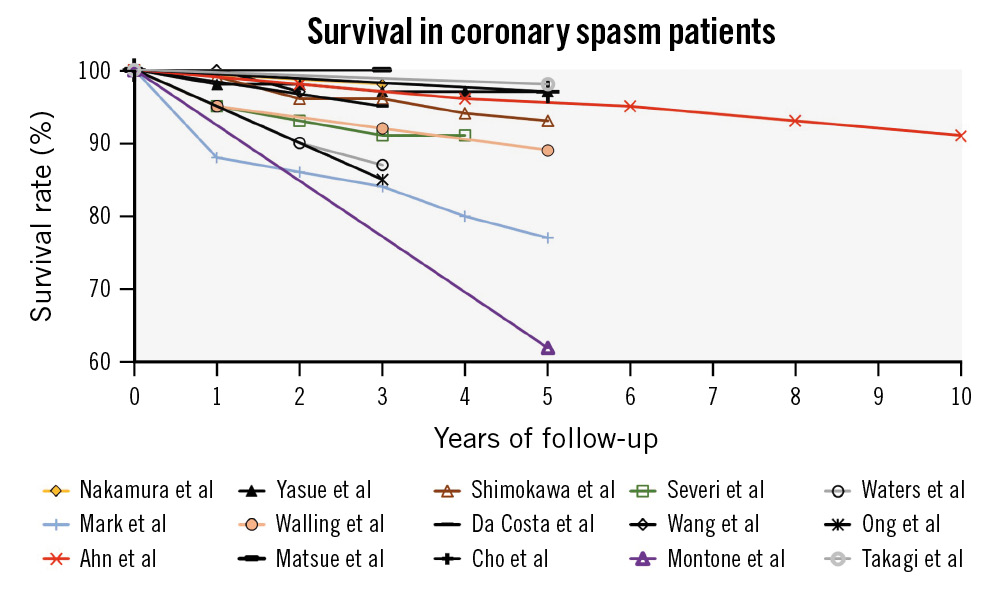
Figure 7. Prognosis of CS patients. Most studies of patients with obstructive CAD (non-black lines) demonstrated significantly worse prognoses than studies of patients without obstructive CAD (black lines). CAD: coronary artery disease; CS: coronary spasm
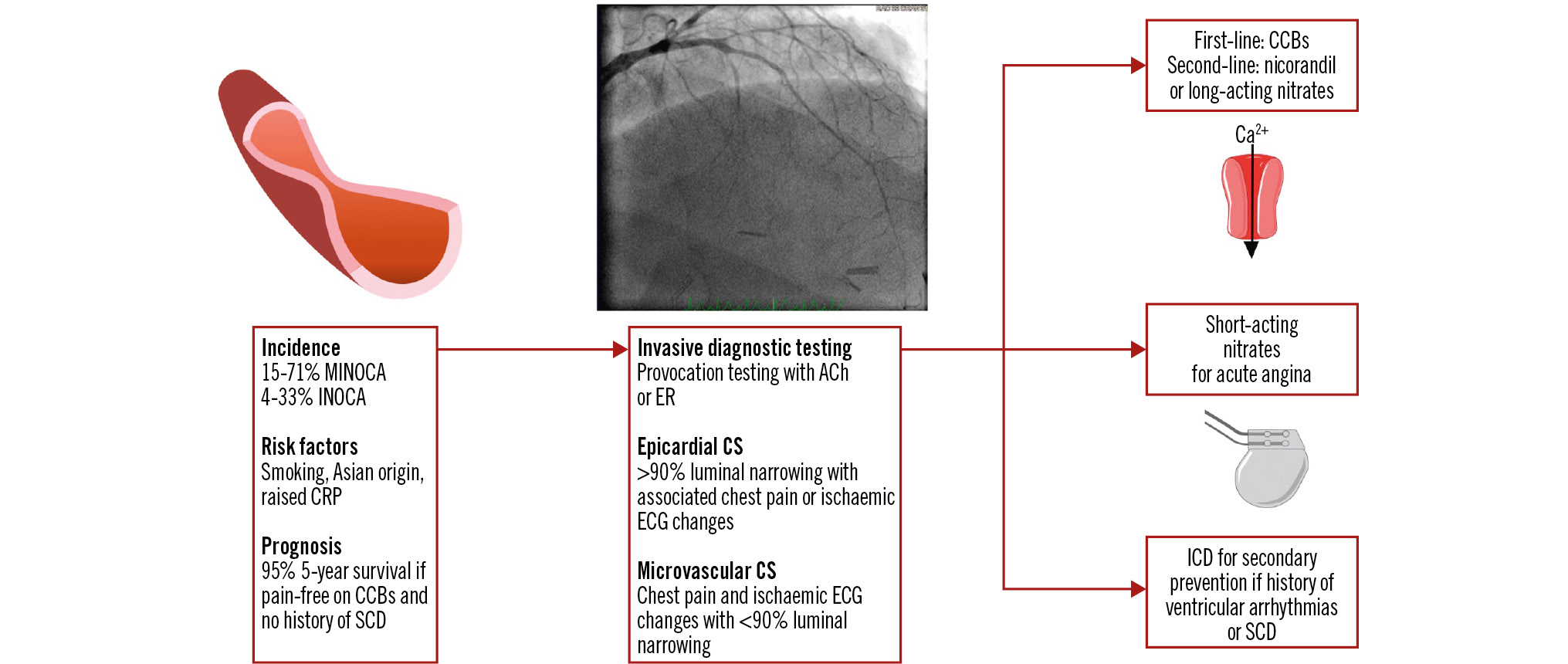
Central illustration. Coronary artery spasm. ACh: acetylcholine; Ca2+: calcium; CCB: calcium channel blocker; CRP: C-reactive protein; CS: coronary spasm; ECG: electrocardiogram; ER: ergonovine; ICD: implantable cardioverter defibrillator; INOCA: ischaemia with non-obstructive coronary arteries; MINOCA: myocardial infarction with non-obstructive coronary arteries; SCD: sudden cardiac death
Clinical challenges and future research directions
Patients who remain chest pain-free with medical therapy generally have excellent long-term outcomes. However, patients with ongoing symptoms remain a therapeutic challenge. Differentiation of microvascular spasm from endothelium-dependent CMD may be helpful. Case studies have reported success with phosphodiesterase-5 inhibitors and guanylate cyclase stimulators69. Further work is needed. The pain clinic can help with quality of life, including the consideration of spinal cord stimulators.
Conclusions
CS is an important cause of chest pain and continues to be underdiagnosed. CS is easier to recognise on provocation testing, while microvascular CS is less defined. Recent studies suggest CS and vasomotor abnormalities are a common cause of MINOCA. Patients with CS treated with CCBs who are rendered pain-free typically have an excellent prognosis; however, the management of patients who are still symptomatic despite maximal available medical therapy remains challenging. Improved recognition is key to appropriate referral for provocation testing and timely diagnosis.
Conflict of interest statement
The authors have no conflicts of interest to declare.

