
CASE SUMMARY
BACKGROUND: A 51-year-old man with recurrent chest pain admitted with NSTEMI developed acute anterolateral STEMI with recurrent VF and prolonged cardiac arrest.
INVESTIGATION: Coronary angiography.
DIAGNOSIS: Anterolateral STEMI.
MANAGEMENT: Percutaneous coronary intervention.
KEYWORDS: coronary angiogram, coronary artery dissection, coronary artery thrombosis, coronary vasospasm, drug-eluting stent, kissing balloon inflation
PRESENTATION OF THE CASE
A 51-year-old male (DT) with an extensive history of ischaemic heart disease (IHD) and previous percutaneous coronary interventions (PCI) was admitted for a second attempt at wide local excision of a lower lip squamous cell carcinoma (SCC), for which his second antiplatelet agent was withheld. Shortly after, he developed a brief episode of chest pain with no electrocardiogram (ECG) changes or cardiac enzyme rise, and was discharged home later that day. That evening, he developed twenty minutes of ischaemic-sounding chest pain associated with a brief loss of consciousness. He was re-admitted for management of a non-ST-elevation myocardial infarction (NSTEMI) as per the acute coronary syndrome (ACS) therapeutic guidelines.
Mr DT was known to be poorly compliant with medical advice and non-adherent to medications. He failed to stop smoking and used intravenous recreational drugs, including amphetamines and cannabis, confirmed by toxicology screening during one of his prior admissions, although he repeatedly denied recent use.
Mr DT has a background of accelerated coronary artery disease (CAD), presenting initially with a NSTEMI 13 months earlier, with his first coronary angiography (CAG) showing a severe lesion of the posterior descending artery (PDA) branch of the right coronary artery (RCA), which was stented with a bare metal stent (BMS) (MULTI-LINK 8™, 2.5×15 mm; Abbott Vascular, Santa Clara, CA, USA), given his poor medication compliance. There was a moderate ostial left anterior descending (LAD) artery lesion that was managed medically. Four months later he suffered an anterior ST-elevation myocardial infarction (STEMI) where his LAD lesion was stented (BMS, MULTI-LINK 8, 4.0×15 mm). He had a new moderate ostial left circumflex artery (LCx) lesion, which was treated medically. Subsequently, he continued to present to our coronary care unit (CCU) with recurrent episodes of chest pain where, at times, repeat CAG showed no significant change. The initial SCC removal attempt was aborted shortly after local anaesthetic administration due to low BP and bradycardia; therefore, a repeat coronary angiogram was performed which showed that the previously noted ostial LCx lesion had progressed. We performed balloon angioplasty only, considering his poor compliance. This occurred two weeks prior to his current presentation.
Following his current admission, Mr DT remained pain-free overnight; however, in the morning, he developed severe crushing central chest pain followed by a decrease in consciousness and complete heart block with a blood pressure of 70/50 mmHg (Figure 1A). This was followed by ventricular fibrillation (VF) for which he received a single direct current shock and 1 mg of IV adrenaline, which stabilised him. He had anterolateral ST elevation (Figure 1B) and therefore underwent an urgent CAG that showed a widely patent previous LAD stent and worsening of his LCx ostial lesion (Figure 2A, Moving image 1). This was stented this time, given his clinical scenario, with a drug-eluting stent (DES) and the procedure was completed with a kissing balloon inflation (KBI) into the LAD and the LCx stents as shown (Figure 2B, Figure 2C) with a good result (Moving image 2).
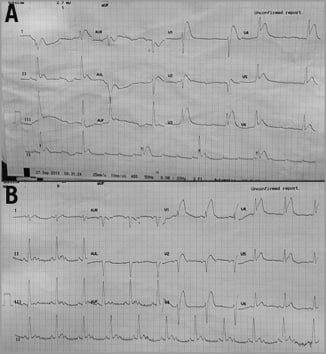
Figure 1. 12-lead ECG. A) Complete heart block; B) anterolateral ST-elevation.
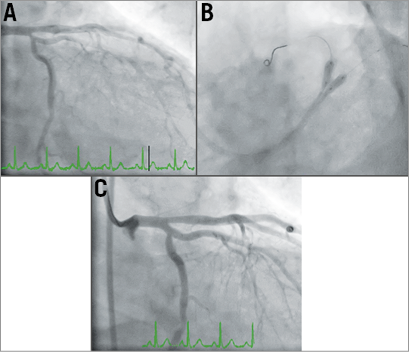
Figure 2. First catheterisation. Severe ostial LCx lesion, normal proximal LAD and patent stent (A), followed by LM-LCx stent insertion (proximal to high marginal branch) stent and KBI (B). Successful angiographic outcome (C).
Within two hours of returning to the CCU, he developed recurrent VF along with anterior ST elevation on his 12-lead ECG (Figure 3), necessitating cardiopulmonary resuscitation with reactivation of the catheterisation laboratory for urgent CAG. Whilst there was evidence of spasm, it was felt that the KBI performed in the PCI from earlier that morning could have resulted in proximal LAD/distal left main (LM) dissection with subsequent thrombosis (Moving image 3), and therefore the LM was stented into the LAD and KBI was used again with satisfactory angiographic results (Figure 4, Moving image 4). However, Mr DT was now intubated and requiring IV inotropic support. We noted that he had a small, stable LM dissection flap following the stent, which did not require further intervention. He was given a bolus followed by IV infusion of the IIb/IIIa glycoprotein inhibitor abciximab, in addition to DAPT. He was transferred to the intensive care unit (ICU).
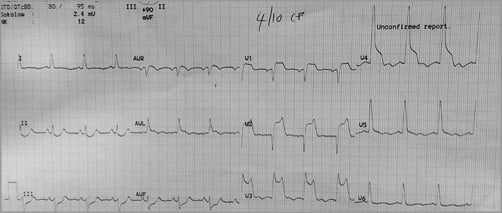
Figure 3. 12-lead ECG. Anterior ST-elevation after VF arrest necessitating second urgent CAG.
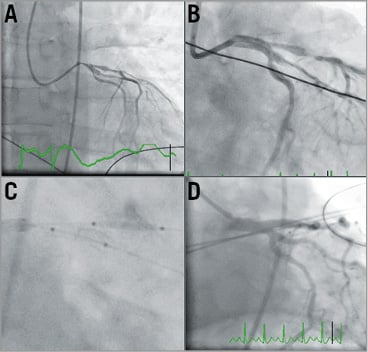
Figure 4. Second catheterisation. A) Poor left coronary perfusion with evidence of coronary spasm. B) Deployed LM stent. C) Angioplasty post stent insertion into LAD and LCx. D) Coronary reperfusion post LM, LAD and LCx stents.
In the ICU, he was started on a noradrenaline and lignocaine infusion along with intra-aortic balloon pump (IABP) insertion due to haemodynamic instability and recurrent ventricular arrhythmias. He was again returned to the catheterisation laboratory where his left coronary angiogram showed widespread coronary artery spasm with poor coronary perfusion (Figure 5A, Moving image 5). He subsequently had no underlying rhythm and CPR did not result in return of output, despite ongoing efforts with both noradrenaline and adrenaline infusions, and 1 mg IV adrenaline bolus every three minutes, as per the current CPR guidelines.
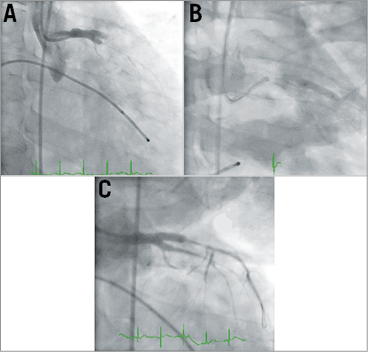
Figure 5. Third catheterisation. A) Absence of flow beyond previously stented LM, LAD and LCx. Unsuccessful balloon angioplasty (B & C).
A temporary transfemoral pacing wire was inserted. A balloon inflation of the recently stented LM, LAD and LCx arteries was performed with no angiographic or haemodynamic improvement (Figure 5B, Figure 5C, Moving image 6). There was no obvious dissection or thrombosis, yet there was diffuse widespread spasm in the coronary arteries, and now with no cardiac rhythm/output there was no electromechanical capture. How should we have proceeded to treat this critically ill young patient who was now in agonal rhythm?
How would I treat?
THE INVITED EXPERTS’ OPINION

This is an interesting and challenging case of a 51-year-old gentleman with recurrent coronary spasms presenting as repeat acute coronary syndromes which ultimately resulted in refractory cardiac arrest. Since the diagnosis of repeat coronary spasms was initially not established, the patient received several stents during the previous 13 months. The trigger for coronary spasms leading to index hospitalisation was probably local anaesthesia for excision of a lower lip carcinoma alone or in combination with intravenous recreational drugs. Coronary spasms leading to refractory cardiac arrest were undoubtedly further facilitated by infusion of norepinephrine and repeat boluses of epinephrine during the intensive care treatment and resuscitative efforts1. We believe refractory cardiac arrest was ultimately caused by a vicious circle with “more catecholamines causing more coronary spasm”. Indeed, coronary angiography during ongoing resuscitation revealed that only proximal stented segments of the left coronary artery (LCA) were patent. Moreover, we could assume that coronary spasm was probably present also on the right coronary artery (RCA) not shown by angiography. As expected, temporary pacemaker, intra-aortic balloon pump and balloon angioplasty did not result in re-establishment of coronary patency which would, in turn, have facilitated re-establishment of spontaneous circulation.
In this situation, we would immediately proceed with implantation of femoral cannulas for rescue veno-arterial extracorporeal membrane oxygenation (VA ECMO) during ongoing chest compression, using our protocol of fluoroscopy-guided emergency implantation in the catheterisation laboratory. After establishment of ECMO flow, we would stop chest compression, completely discontinue all catecholamines and adjust ECMO flow to maintain mean arterial pressure between 60 and 70 mmHg. After haemodynamic stabilisation, we would proceed with repeat coronary angiography of both LCA and RCA, and administer intracoronary boluses of nitroglycerine (200-300 micrograms) if coronary spasms were still present. After re-establishment of coronary patency, we would avoid any catecholamines, use nitroglycerine infusion and start calcium channel blockers. Since the patient would still be on ECMO, possible nitroglycerine-induced hypotension could be easily compensated for by increasing retrograde flow. If the patient did not regain consciousness after re-establishment of cerebral perfusion, we would implement mild therapeutic hypothermia (32-34 degrees C) for 24 hours using ECMO circuit for induction, maintenance and rewarming2. After completion of hypothermia and expected improvement of left ventricular function, we would wean the patient off ECMO and ask the vascular surgeon to remove the cannulas.
If the patient survived this critical event, it should be noted that he would remain at higher long-term risk compared to patients with vasospastic angina without cardiac arrest3. Discontinuation of any recreational drugs and smoking would be absolutely essential. Special caution would be needed if either local or systemic anaesthesia were used in the future. We would recommend aggressive medical therapy with long-acting nitrate and calcium channel blockers, and an implantable cardioverter-defibrillator3. Because of the bifurcational left main stenting with three stents, we would recommend dual antiplatelet therapy for at least 12 months and closely follow the patient for possible occurrence of in-stent restenosis.
Conflict of interest statement
M. Noc has received a speaker’s honorarium from the Maquet Getinge Group (Rastatt, Germany). M. Fister has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

The clinical scenario consists of a 51-year-old male (DT) with an extensive history of ischaemic heart disease (IHD), diffuse atherosclerotic coronary artery disease and previous percutaneous coronary interventions (PCI), non-compliant with secondary prevention and non-adherent to medications. He failed to stop smoking and uses intravenous recreational drugs, including amphetamines and cannabis.
Initially, the patient suffered an acute coronary syndrome without ST-segment elevation, with a balloon-only mechanical intervention on the proximal circumflex coronary artery (CFX). What followed was an escalation of ischaemic events and flow-limiting complications requiring repeat interventions which resulted in additional stenting of the CFX and the left main coronary artery (LM) into the left anterior descending artery (LAD). A small dissection distal from the stent in the LM was left untreated. The patient received a bolus followed by IV infusion of a glycoprotein IIb/IIIa inhibitor (abciximab), in addition to DAPT. Intra-aortic balloon counterpulsation was implemented as a haemodynamic support in combination with vasopressors (noradrenaline). In the ICU, he was started on a lignocaine infusion for recurrent ventricular arrhythmias. The course of events seemed to come to a fatal end in the catheterisation laboratory once again. A repeat left coronary angiogram showed widespread coronary artery spasm (CAS) with a poor myocardial perfusion and no obvious dissection or thrombosis. The patient developed an agonal rhythm and no cardiac output. Resuscitation was started.
At this stage the first concern is to keep the patient alive. In the absence of any mechanical complications following STEMI, excluded by an emergency transthoracic echocardiography, rapid percutaneous insertion of a (mini-) extracorporeal life support (ECLS) taking advantage of the catheterisation laboratory facilities is the next step. ECLS is preferred over other systems of mechanical support (e.g., Impella® platform; Abiomed, Danvers, MA, USA) as it can provide near complete respiratory and circulatory support, independent of the intrinsic cardiac rhythm or ventricular function. The IABP can be left in place to provide unloading of the left ventricle and as an attempt to maintain coronary perfusion4,5.
The second step is to correct for any acid-base and electrolyte disorders as they may affect the effect of pharmacological agents including inotropes and vasopressors.
The coronary perfusion should be optimised while treating potential remaining fixed flow-limiting lesions. After optimising the patient’s anticoagulation status (aiming for a haemachrome activated clotting time of 225-250 s in the presence of abciximab), intracoronary imaging with optical coherence tomography of the left main and LAD coronary artery may be considered.
Coronary artery spasm (CAS) following the initial PCI may have triggered the clinical presentation in this patient. His clinical history (heart block, ventricular fibrillation [VF]) and ECG (Figure 1B) were already pointing in that direction (rather than to an isolated CFX problem). Vascular hyperactivity (spontaneous or following coronary manipulation) is thought to be the underlying autogenic mechanism for spasm. Intracoronary nitroglycerine injections (up to 200 mcg) may promptly alleviate episodes of spasm and restore coronary perfusion without a significant reduction in systemic blood pressure6. Norepinephrine may contribute to CAS and should be down-titrated when possible7. Long-acting nitrate preparations, nicorandil or dual or multiple calcium blocking agent treatment reduce the frequency of recurrent events8,9. Combined treatment with an implantable cardioverter-defibrillator (ICD) may be required in such patients, but reasonable risk stratification needs to be established first. Smoking cessation and abstinence of illicit drugs (e.g., cocaine, cannabis) is a must10,11.
In conclusion, while CAS is not an uncommon event and spontaneous spams might occur in young patients as well as during percutaneous coronary intervention in elderly patients, extensive multivessel spasm rarely occurs. Recognising and rescuing massive multivessel epicardial CAS is paramount, because it can cause devastating haemodynamic embarrassment and even death. Ventricular fibrillation is an important cause of sudden cardiac death in patients with coronary vasospasm. Intensive medical treatment against coronary vasospasm is most important for prevention of VF recurrence!
Conflict of interest statement
P. Vranckx reports receiving speaking or consulting fees from Bayer Healthcare, Daiichi-Sankyo, and AstraZeneca outside the submitted work. M. Valgimigli reports grants from The Medicines Company, grants from Terumo, during the study; grants from AstraZeneca, and personal fees from Terumo, St. Jude Vascular and Abbott Vascular, outside the submitted work. H. Heidbuchel reports receiving consulting fees as a member of the scientific advisory boards for Boehringer-Ingelheim, Bayer, BMS-Pfizer, Daiichi-Sankyo and Cardiome, and receiving lecture fees from these companies.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Mr DT posed an extremely challenging management dilemma given his ongoing instability and high likelihood of death, especially during the last coronary intervention where he had no output and loss of electromechanical capture to ventricular pacing, despite having had high doses of IV adrenaline and noradrenaline infusions. He was being actively resuscitated, with chest compressions and intravenous adrenaline boluses, in addition to multiple coronary interventions, as previously described.
He had a severe, diffuse coronary spasm and, whilst this can occur in 1-5% of patients during percutaneous coronary intervention (PCI)12, it is most likely that his amphetamine use was the precipitating factor, as this is a known mediator of coronary vasospasm. Mr DT subsequently admitted to using both amphetamines and cannabis during his hospital stay and prior to his event early in the morning. It is also likely that the noradrenaline infusion had an additive effect and when it was ceased we noted some mild improvement in coronary flow. We had initially thought that ongoing hypotension and coronary spasm had resulted in thrombosis of his deployed stents, and therefore we revascularised his LMCA, LAD and circumflex arteries (Figure 5A, Figure 5B). Despite that, he had ongoing diffuse coronary spasm, and repeat balloon angioplasty failed to resolve the problem (Figure 5C, Moving image 6). The use of intracoronary glyceryl trinitrate and adenosine, both of which can relieve coronary vasospasm, was not thought possible owing to his clinical state (cardiogenic shock) and subsequent agonal rhythm.
As a final manoeuvre, he was given intracoronary adrenaline (1 mg 1:1,000 adrenaline) as a bolus, with the resulting angiogram (Figure 6, Moving image 7) showing complete resolution of the angiographic appearance of spasm. His output returned and he was transferred in a stable condition to the ICU.
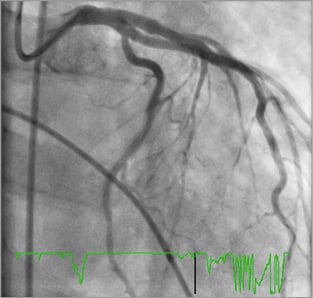
Figure 6. Successful outcome of intracoronary adrenaline with LMCA angiography showing resolution of angiographic appearance of spasm.
Intravenous adrenaline causes peripheral vasoconstriction and has a positive cardiac inotropic effect. Coronary arteries have both alpha and beta adrenergic receptors; however, in the smaller coronary vessels, β2 adrenergic receptors predominate13. This difference in adrenergic receptor density is the reason why intracoronary adrenaline causes coronary vasodilatation. The use of intracoronary adrenaline has been studied in slow coronary reflow phenomena post PCI14,15, but its use is not standard owing to the paucity of clinical data. To our knowledge, our case is the first documenting the use of intracoronary adrenaline as a resuscitative measure during coronary angioplasty for diffuse coronary vasospasm.
Mr DT was discharged home two weeks later and, whilst he has returned with another episode of chest pain, a repeat coronary angiogram showed patent coronary stents.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Left coronary artery angiogram demonstrating severe ostial LCx disease.
Moving image 2. Successful angiographic outcome of LM-LCx stent insertion.
Moving image 3. Poor left coronary perfusion. Possible LM/LAD dissection/thrombosis. Evidence of diffuse spasm.
Moving image 4. Successfully stented LM, LAD and LCx.
Moving image 5. Absence of coronary flow beyond LM, LAD and LCx stented segments.
Moving image 6. Poor coronary reperfusion despite repeat balloon angioplasty of LM, LAD and LCx.
Moving image 7. Successful outcome of intracoronary adrenaline with LMCA angiography showing resolution of angiographic appearance of spasm.
Supplementary data
To read the full content of this article, please download the PDF.
Left coronary artery angiogram demonstrating severe ostial LCx disease.
Successful angiographic outcome of LM-LCx stent insertion.
Poor left coronary perfusion. Possible LM/LAD dissection/thrombosis. Evidence of diffuse spasm.
Successfully stented LM, LAD and LCx.
Absence of coronary flow beyond LM, LAD and LCx stented segments.
Poor coronary reperfusion despite repeat balloon angioplasty of LM, LAD and LCx.
Successful outcome of intracoronary adrenaline with LMCA angiography showing resolution of angiographic appearance of spasm.

