Abstract
Aims: The aim of this study was to systematically analyse the available data from trials comparing revascularisation by drug-eluting stent (DES) placement versus coronary artery bypass grafting (CABG) in patients with multivessel coronary artery disease (CAD).
Methods and results: We searched PubMed, Medline and several internet sources for randomised controlled trials comparing DES placement to CABG in patients with multivessel coronary artery disease. There were no restrictions on journal type or population studied. Prior to data collection we chose to analyse the prospectively performed trials separately from data obtained retrospectively. Four prospective trials were identified which enrolled a total of 3,895 patients: 1,914 in the DES arm and 1,981 patients in the CABG arm. Pooled analysis of data from these four studies showed that in patients treated DES compared to CABG there was a similar risk of the combined endpoints of death, myocardial infarction and stroke (10.2% versus 10.8%, respectively; RR=0.94 [95% CI=0.77-1.116]; p=0.56), but a significantly higher risk of target vessel revascularisation (TVR) (14.6% versus 6.8%, respectively; RR=2.09 [95% CI=1.72-2.55]; <0.001) and, therefore, a significantly higher risk of MACCE (21.2% versus 16.3%, respectively; RR=1.27 [95% CI=1.09-1.48]; p=0.002). Interestingly, when MACCE rates at one year are used for these trials the risk is equivalent between DES and CABG (14.4% versus 12.5%, respectively; RR=1.05 [95% CI=0.70-1.57]; p=0.83). Analysis of observational data revealed similar findings.
Conclusions: Overall, PCI with DES placement was safe in patients with multivessel disease compared to CABG, but is associated with a significantly higher risk of TVR.
Introduction
Current ACC/AHA guidelines recommend that patients with multivessel coronary artery disease undergo revascularisation with coronary bypass grafting (CABG)1. However, improvements in percutaneous coronary intervention (PCI) technique and materials have lead to several recent studies which challenge these recommendations. Randomised trials have shown that PCI with bare metal stenting (BMS) results in similar morbidity and mortality to CABG, at least among the relatively low-risk patients enrolled in these trials2,3. In these studies PCI results in a higher risk of repeat target vessel revascularisation (TVR)2. Since the advent of the drug-eluting stent (DES) these trials may well be obsolete, since it is well established that DES placement results in decreased need for TVR compared to BMS placement4,5. Data from prospective, randomised trials comparing DES placement to CABG in patients with multivessel coronary artery disease (CAD) are needed and are beginning to appear in the literature including the ERACI III trial (Argentine Randomized Trial of Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Patients With Multiple Vessel Disease)6, the SYNTAX Trial (Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease)7, the CARDia Trial (Coronary Artery Revascularization in Diabetes)8 and the ARTS II trial (Arterial Revascularization Therapies Study)9. Due to sample size and follow-up limitations none of these trials can be considered definitive, therefore, the goal of this study is to systematically analyse the available data from prospective trials comparing revascularisation by DES placement versus CABG in patients with multivessel coronary artery disease. Also, because the majority of data available comparing DES to CABG for the treatment of multivessel disease are retrospective analyses, a review of these registries is necessary. We hypothesised that DES placement in patients with multivessel coronary artery disease will have equivalent outcomes compared with CABG surgery regardless of the risk of repeat TVR.
Methods
Criteria for study selection
We restricted our meta-analysis to randomised trials and observational studies comparing drug-eluting stent (DES) placement to coronary artery bypass grafting (CABG) in patients with multivessel coronary artery disease. There were no restrictions on journal type or population studied. Prior to data collection we chose to analyse data from prospectively performed trials separately from studies that utilised retrospectively analysed registries of patients.
Data sources
We searched Medline and PubMed using the search terms: drug-eluting stent, DES, coronary artery bypass, coronary artery bypass graft, CABG and multivessel. In addition, relevant reviews from major medical journals were identified and assessed for possible information on trials of interest. Internet based sources of information on the results of clinical trials in cardiology (http://www.theheart.org, http://www.tctmd.com, www.clinicaltrials.gov and Google Scholar) were also searched. The search was conducted by two independent investigators (AMF and FJA), discrepancies were resolved by consensus. Furthermore, because of the well established association between survival and revascularisation among diabetic patients with multivessel disease10 and recent data from the BARI 2D Trial (The Bypass Angioplasty Revascularization Investigation 2 Diabetes)11 we analysed the subset of patients with diabetes mellitus.
Statistical analysis
All meta-analyses were performed with Review Manager software (RevMan® Analyses Version 5.0.4, Copenhagen, Denmark; The Nordic Cochrane Center, The Cochrane Collaboration, 2008). The primary outcome was major adverse cardiovascular and cerebral vascular events (MACCE) rates for patients treated with drug-eluting stents (DES) versus those treated with coronary bypass grafting (CABG). The M-H risk (RR) and its 95% confidence interval (CI) for each study were first computed using the random-effects model since relative weights assigned under random effect will be more balanced than those assigned under fixed effect. Thus, as described by DerSimonian et al the random-effects model incorporates any amount of heterogeneity in the analysis of the overall efficacy of the treatment12. Heterogeneity between studies was analysed by Q statistics, i.e., weighted sum of squared deviations, and Q has a χ2 distribution with (# of studies-1) as its degrees of freedom. This described the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error. Funnel plot was plotted to show the treatment effect against a measure of study size; it was a visual aid to detecting bias or systematic heterogeneity. Egger’s linear regression model quantified the bias captured by the funnel plot and the bias was captured by the intercept; it had a student t-distribution with (# of studies -2) degrees of freedom.
Results
Using the search criteria outlined we identified four prospective studies for our main analysis. Figure 1 shows that there is little publication bias among these studies. The SYNTAX Trial (Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease) with one year7 and two year results13 and the CARDia Trial (Coronary Artery Revascularization in Diabetes)8 were randomised, controlled trials. The ARTS II (Arterial Revascularization Therapies Study)9 and the ERACI III trial (Argentine Randomized Trial of Coronary Angioplasty with Stenting Versus Coronary Bypass Surgery in Patients with Multiple Vessel Disease)6 were prospective trials designed as non-randomised extensions of randomised, controlled trials. The ERACI III and ARTS II trials evaluated DES placement in consecutive patients who would have met criteria for stent placement in their associated randomised, controlled trial and made comparisons between DES placement versus CABG in patients with multivessel coronary artery disease.
Patient characteristics for each of these four studies are shown in Table 1. Among these four trials a total of 3,895 patients were included: 1,914 in the DES arm and 1,981 patients in the CABG arm. Follow-up time ranged from one to three years for the four studies included. Forty-two percent (42%) of the patients who received DES were diabetic compared to 44% who underwent CABG. In three of the studies (SYNTAX, ARTS II and ERACI III) data was available on age, gender and acute coronary syndromes. In these three studies the average age ranged from 63 to 65 years for patients who received DES and from 61 to 65 years in patients who underwent CABG. Seventy percent (70%) of patients who received DES were male compared to 68% of patients who underwent CABG. The percentage of patients presenting with acute coronary syndrome was 34% for both groups.
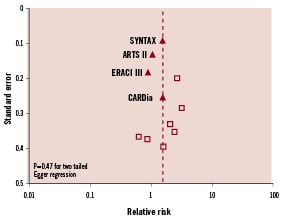
Figure 1. Funnel plot of studies.
Pooled analysis of data from these four studies shows there were similar rates of death in patients who underwent PCI with DES compared to CABG (4.9% versus 5.0%, respectively; RR=0.90 [95% CI=0.60-1.37]; p=0.63), myocardial infarction (5.2% versus 4.4%, respectively; RR=1.12 [95% CI=0.73-1.74]; p=0.60), stroke (1.9% versus 2.7%; respectively; RR=0.77 [95% CI=0.38-1.58]; p=0.48) and a similar risk of the combined endpoints of death, myocardial infarction and stroke (10.2% versus 10.8%, respectively; RR=0.94 [95% CI=0.77-1.116]; p=0.56) (Figure 2). There were higher rates of target vessel revascularisation (TVR) among patients who underwent DES placement versus CABG (14.6% versus 6.8%, respectively; RR=2.09 [95% CI=1.72-2.55]; <0.001) and a higher risk of MACCE (21.2% versus 16.3%, respectively; RR=1.27 [95% CI=1.09-1.48]; p=0.002) (Figure 3).
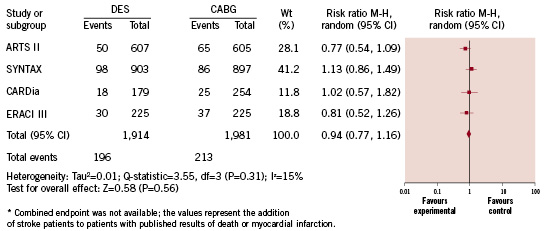
Figure 2. Risk of death, myocardial infarction or stroke.
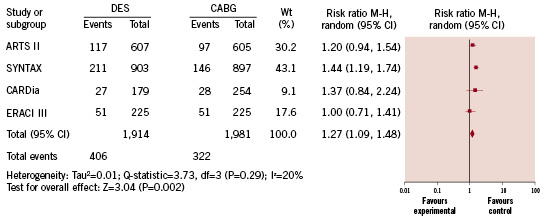
Figure 3. Risk of major adverse cardiac and cerebral vascular events.
Sub-group analyses of the prospective trials
Data on outcomes among patients with diabetes mellitus was available in all studies including the SYNTAX trial at one year14. Overall, 782 diabetic patients underwent DES placement and 826 underwent CABG. Outcome data on TVR and MACCE was available in all studies and the combined endpoint of death, MI or stroke was available for analysis in all studies except the ERACI III Trial. Among this sub-group of diabetic patients, those treated with DES placement had a similar risk of the combined endpoint of death, myocardial infarction or stroke (8.0% versus 8.1%, respectively; RR=0.99 [95% CI=0.71-1.39]; p=0.96). On the other hand DES patients had higher MACCE rates compared to CABG patients (18.8% versus 10.4%, respectively; RR=1.69 [95% CI=1.23-2.31]; p=0.001) due to a significantly higher rate of TVR (14.1% versus 4.1%, respectively; RR=2.99 [95% CI=1.87-4.77]; p<0.001).
We re-analysed the outcomes of the ERACI III, ARTS II, SYNTAX and CARDia trials using only the patients with 3-vessel disease among the SYNTAX trial participants. Among this group of patients the MACCE rates were 20.9% versus 16.0% for DES versus CABG treatment, respectively (RR=1.27 [95% CI=1.06-1.52]; p=0.01). The rates of death, myocardial infarction or stroke were 10.2% versus 10.5% for DES versus CABG treatment, respectively (RR=0.96 [95% CI=0.72-1.29]; p=0.81).
We also analysed the one year outcomes of all patients from the ERACI III, ARTS II, SYNTAX and CARDia trials which revealed that MACCE rates were equivalent between DES and CABG (14.4% versus 12.5%, respectively; RR=1.05 [95% CI=0.70-1.57]; p=0.83) (Figure 4), despite a higher rate of TVR among patients who underwent DES placement (10.9% versus 4.2% for DES versus CABG treatment, respectively; RR=2.25 [95% CI=1.78-2.85]; p<0.001). The rate of stoke was 0.8% versus 2.0% for DES versus CABG treatment, respectively (RR=0.47 [95% CI=0.16-1.33]; p=0.15). The rate of TVR was 10.9% versus 4.2% for DES versus CABG treatment, respectively (RR=2.25 [95% CI=1.78-2.85]; p<0.001).
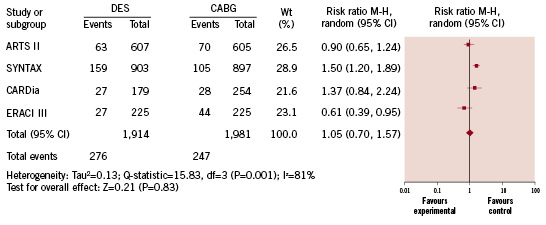
Figure 4. Risk of major adverse cardiac and cerebral vascular events at one year.
Analysis of observational studies
We identified 11 observational studies which evaluated DES placement versus CABG in patients with multivessel disease and have data on outcomes15-25. In total, 17,333 patients with multivessel disease were included; 7,095 patients received DES and 10,238 underwent CABG as shown in Table 2. Figure 1 shows that there is little publication bias among these trials. As shown in Figure 5, the pooled outcomes in these patients reveals that DES placement was associated with a similar risk of death compared to CABG (5.6% versus 5.9%, respectively; RR=1.18 [95% CI=0.80-1.75]; p=0.39). Patients treated with DES placement had higher risk of myocardial infarction (2.5% versus 1.8%, respectively; RR=1.56 [95% CI=1.22-1.98]; p<0.001), target vessel revascularisation compared to CABG (11.3% versus 1.6%, respectively; RR=6.44 [95% CI=3.59-11.57]; p<0.001) and MACCE rates (7% versus 5%, respectively; RR=1.78 [95% CI=1.24-2.55]; p=0.002), but a non-significant decreased risk of stroke (0.7% versus 1.3%, respectively; RR=0.60 [95% CI=0.33-1.07]; p=0.08).
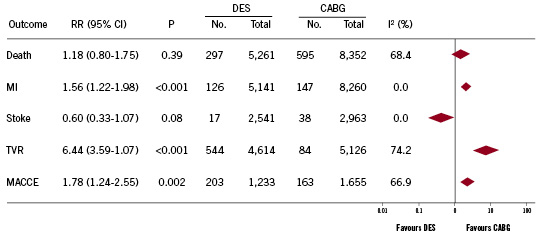
Figure 5. Pooled analysis of outcomes from observational studies.
Sensitivity analyses
We repeated the analysis of MACCE and stroke rates among the observational studies after adding data from the two prospective non-randomised trials (ARTS II and ERACI III), however there was no significant change to the observed outcome. MACCE rates 4.7% versus 2.8% for DES versus CABG treatment, respectively (RR=1.57 [95% CI=1.16-2.13]; p=0.003). Stroke rates 0.5% versus 0.5% for DES versus CABG treatment, respectively (RR=0.80 [95% CI=0.53-1.20]; p=0.28).
We also repeated the analysis of MACCE rates among the four prospective studies after removing the non-randomised trials (ARTS II and ERAC III). After removal of these studies there was no change in the observed result (12.4% versus 8.8% for DES versus CABG treatment, respectively; RR=1.43 [95% CI=1.20-1.70]; p<0.001).
Discussion
We present a systematic review of pooled prospective studies evaluating drug-eluting stent (DES) placement compared to coronary artery bypass grafting (CABG) for multivessel coronary artery disease. Compared to CABG, treatment of multivessel coronary disease with DES placement results in: 1) a similar risk of the combined endpoint of death, myocardial infarction or stroke, 2) a higher risk of target vessel revascularisation (TVR) and 3) a higher risk of major adverse cardiac events (MACCE), driven by differences in TVR. Subgroup analysis of diabetic patients shows similar findings and the pooled analysis of observational studies shows similar MACCE rates but a decreased (though non-significant) risk of stroke for patients treated with DES placement.
Results from the prospective randomised trials
In this pooled analysis the risk of adverse events was statistically equivalent between DES placement and CABG, with the exception of target vessel revascularisation. A careful examination of the data reveals that the combined endpoint of death, myocardial infarction or stroke was decreased by 1% among patients treated with DES placement, but we were unable to establish statistical significance due to limitations in study size and power. In order to show a significant difference in this outcome a much a larger study or pooled analysis of studies would be necessary. If we assume the risk of death, MI or stroke is 8.7% for patients treated with DES placement and 9.7% for CABG (as demonstrated by the above analysis) a two group continuity corrected χ2 test with 80% power requires 13,312 patients for each study group (a total study size of 26,624 patients) to show a significant difference in risk (i.e., a 0.05 two-sided significance level). Several trials are currently recruiting patients: The VA CARDS26, FREEDOM27 (with planned completion in the year 2012) and a randomised, controlled study of DES placement versus CABG in left main disease28. Though these studies will be pivotal in determining the best treatment modality of multivessel disease their combined patient populations (n=2,990 estimated patient enrolment from all three studies) would still not allow for statistical significance to be established for the 1% difference in the combined outcome of myocardial infarction, death or stroke.
Results from the observational studies
Retrospective studies often contain co-founding variables (e.g., operator experience or patient selection) for which there is no adjustment and are susceptible to selection bias. For instance, compared to the SYNTAX trial where participants treated with DES or CABG were well matched by preprocedural co-morbid disease, patients treated with DES from one of the largest observational studies published by Park and colleagues18 more often presented with unstable angina and were more often diabetic compared to patients treated with CABG. However, findings from randomised controlled trials sometime lack real-world applicability. Randomised studies typically exclude patients at high risk of periprocedural complications (e.g., the very elderly, those with renal disease or cardiogenic shock). Observational studies, though fraught with biases, include all-comers and trends in treatment preference that better reflect physician preferences and experiences. Our review of observational studies reveals that carefully selected patients treated with DES placement have relatively similar outcomes to those treated with CABG and these outcomes are predicted by randomised trial results.
Consideration of patient safety and preference
In both the prospective randomised trials and the observational studies DES placement is associated with a slightly lower risk of stroke compared to CABG (1.5% versus 2.4% risk of stroke, respectively for patients from randomised trials [p=0.38] and 0.7% versus 1.3%, respectively for patients from the observational studies [p=0.08]). In fact, despite the lower risk of TVR with CABG, well informed patients sometimes chose PCI rather than CABG to avoid large sternotomy incisions, or due to the fear of stroke or other neurological compromise from cardiopulmonary bypass29. Though a previous meta-analysis of bare metal stenting versus CABG in patients with multivessel disease by Daemon et al showed no difference in the rate of stroke (2.5% vs. 2.9% in the PCI with bare metal stent and CABG groups respectively; p=0.54)30 other studies have shown that the risk of neurological compromise is higher after CABG versus PCI in the immediate post procedure time period31-33. Thus, treatment must be individualised for each patient and always include a discussion of risks, including the risk of TVR and stroke. Though the ACC/AHA guidelines currently recommend CABG for multivessel coronary disease the recently published appropriateness criteria for revascularisation state that the utility of PCI in 3-vessel disease is uncertain34. Thus, it may be reasonable and appropriate to consider PCI with DES in patients from who informed consent is obtained.
Study limitations
The major limitation of our study is that the results are based on the combined data of many heterogeneous prospective trials of which two (ARTS II and ERACI III) were not concurrent, randomised, controlled trials and the trials utilised both paclitaxel and sirolimus coated stents in slightly different populations of patients. However, pooling the results provides more statistical power on which to base conclusions.
Conclusions
This analysis of available data from prospective randomised and observational studies suggests that DES placement compared to CABG in multivessel disease is associated with a similar risk of the combined endpoint of death, myocardial infarction or stroke, but higher risk for TVR and therefore higher risk of MACCE. Overall, PCI with DES placement is safe in patients with multivessel disease compared to CABG, but treatment must be individualised for each patient based on their preprocedure co-morbidities and their understanding of procedural risks.

