Abstract
Aims: Controversy exists about the safety and efficacy of drug-eluting stents (DES) in saphenous vein bypass grafts (SVGs). The aim of this study was to perform a meta-analysis of all published studies comparing DES and bare-metal stents (BMS) in patients with SVGs disease.
Methods and results: We included 22 studies comparing DES versus BMS in 5,543 patients with SVGs disease. The primary efficacy endpoint was target vessel revascularisation (TVR). The primary safety endpoint was mortality. Other outcomes of interest were cardiac mortality, myocardial infarction, target lesion revascularisation (TLR), stent thrombosis and a combined of major adverse cardiac events (MACE). DES significantly reduced the risk of TVR, OR=0.56 (95% CI, 0.41-0.76, p=0.0003) and TLR, OR=0.58 (95% CI, 0.41-0.81; p=0.001). Total mortality and cardiac mortality were significantly lower in DES versus BMS, OR=0.69 (95% CI, 0.49-0.98, p=0.04) and OR=0.71 (95% CI, 0.51-0.99; p=0.04), respectively. The overall risk of stent thrombosis, and myocardial infarction were not significantly different for patients receiving DES vs. BMS. Total MACE were significantly lower in patients receiving DES, OR=0.55 (95% CI, 0.42-0.71; p<0.00001).
Conclusions: This meta-analysis suggests that the use of DES in patients with SVG lesions is associated with a reduction of the need of reintervention and mortality compared with BMS.
Introduction
Drug eluting stents (DES) have demonstrated to reduce restenosis, and the need for subsequent revascularisation procedures of the target vessel, by means of reducing the degree of neo-intimal hyperplasia when treating native coronary arteries1. However, controversy still remains regarding the safety and efficacy of DES in saphenous vein aortocoronary bypass grafts (SVGs). Unlike native coronary arteries, degenerated SVGs usually show diffuse and friable atherosclerotic plaques characterised by abundant lipid debris that tend to embolise during percutaneous coronary intervention (PCI)2. Also the mechanism of in-stent restenosis in SVGs may differ from that of native coronary arteries with delayed endothelialisation, accelerated atherosclerosis and profound inflammation and thrombotic tendency3. These factors could partially mitigate the beneficial effect of DES.
Several small observational studies and a very few randomised clinical trials evaluating DES outcomes in SVGs after up to one year follow-up showed a net beneficial effect compared with bare-metal stent (BMS), mainly reducing the risk of reintervention4-12. However, some concerns have been raised regarding to the safety of DES in patients with SVGs disease. Data from a recent small randomised clinical trial, DELAYED RRISC13, a secondary post hoc analysis of the RRISC trial, have suggested that DES may be associated with an increased rate of mortality, and late stent thrombosis at an average of three years of follow-up. Also very recently, results of other observational studies have been reported with contradictory conclusions regarding the use of DES in SVGs14-18.
All these observational studies and randomised clinical trials had insufficient power to assess the risk of rare complications, such as mortality. For this reason, we performed a meta-analysis from 22 studies comparing DES with BMS to evaluate the safety and efficacy of DES in patients with SVGs disease.
Methods
Selection of the studies
In order to identify the studies to be included, we conducted a computerised bibliographic search of the MEDLINE database (National Library of Medicine, Bethesda, MD, USA) until December 2009. We selected all the studies that compared DES and BMS in patients with SVGs stenosis and provided clinical follow-up data. Various combinations of the following keywords were used: saphenous, vein graft, coronary artery bypass graft, stent, drug-eluting stent, bare-metal stent, coronary surgery. All potentially relevant articles were independently reviewed according to the following inclusion criteria by two investigators (A.S-R and S. J-V) to establish eligibility for the meta-analysis: 1) study that had comparison between DES and BMS in patients with SVG disease; 2) mean duration of follow-up of at least six months; 3) reporting clinical events. Disagreements were resolved by discussion with a third reviewer (RM). When more than one article originated from the same centre, the study that reported more patients and more follow-up was included.
The flow chart of the strategy and selection of studies is depicted in Figure 1. A total of 23 studies were identified. One study was excluded because a study from the same institution was reported later with more patients and follow-up19.
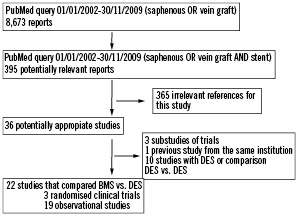
Figure 1. Flowchart of selected studies.
Study outcome
The primary efficacy endpoint of this meta-analysis was the need of reintervention of the target vessel (TVR). The primary safety endpoint was total mortality. Other outcomes of interest were cardiac death, myocardial infarction, target lesion revascularisation (TLR), stent thrombosis, and a combined endpoint of major adverse cardiac events (MACE). The event definitions used in individual studies are given in Table 1.
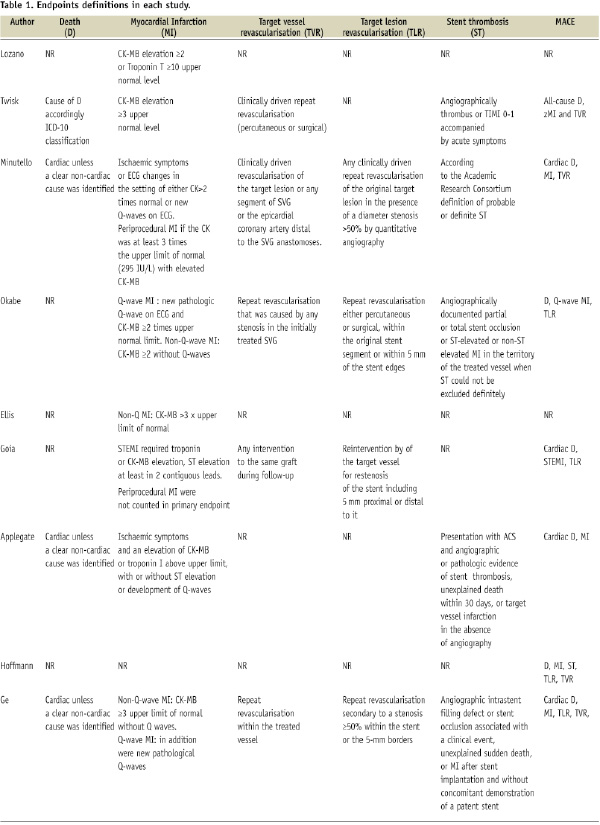
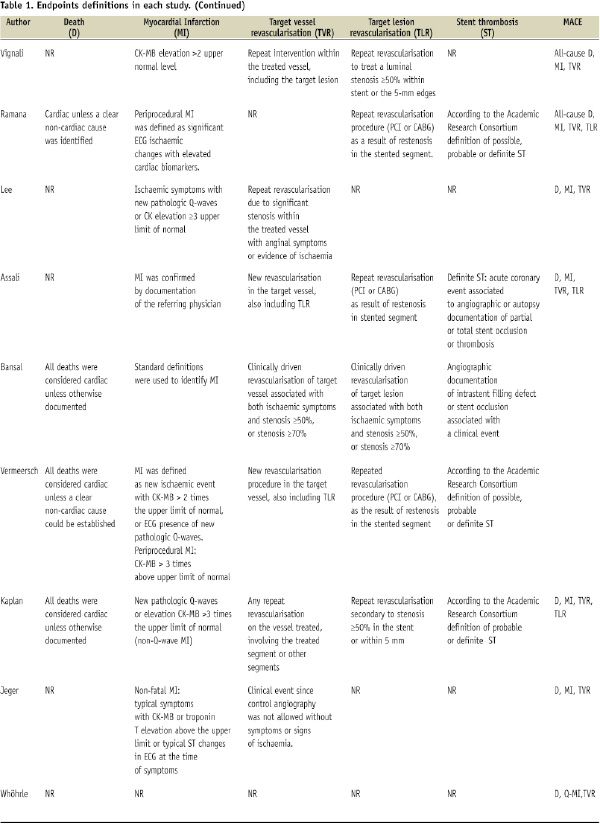

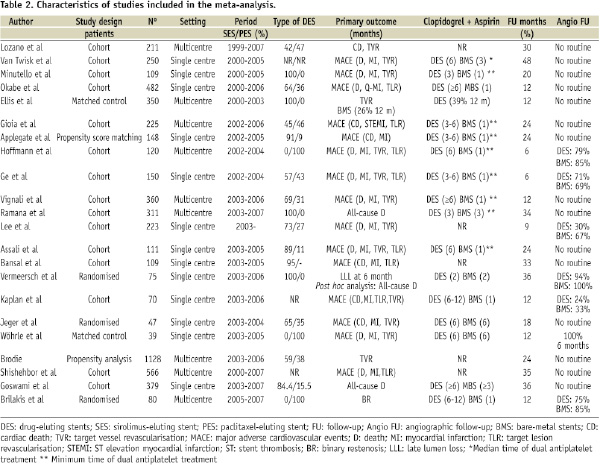
Table 2 shows the number of patients included in each study, as well as the period of time, type of DES, primary outcome of the study, antiplatelet therapy duration, clinical and angiographic follow-up.
Statistical analysis
Review Manager 5.0 (The Cochrane Collaboration, The Nordic Cochrane Centre 2008, Copenhagen, Denmark) was used. Odds ratios (OR) for binary end-points and 95% confidence intervals (CI) were calculated by comparing DES with BMS rates using raw data for each study and for the pooled population. The review was conducted according to the meta-analysis of observational studies of observational studies in epidemiology (MOOSE) recommendations20. Heterogeneity among studies was assessed by the Q test and, as this test is not good enough when evaluating a small number of studies, the level of inconsistency was also assessed by I2 test21,22. Although heterogeneity was not found for mortality, a number of sources of heterogeneity were anticipated a priori, therefore, Der Simonian and Laird random effects model was used to estimate summary measures and their 95% CI, making an adjustment to the study weights according to the extent of variation based on the inverse variance approach. The effect of each study was weighted for its number of patients. A sensitivity analysis was performed according the clinical follow-up (equal or shorter than one year of follow-up vs. longer than one year), population size (<200 vs. >200 patients) and study quality (randomised vs. observational). Null hypothesis was rejected by a type I error minor than 0.05 (α<0.05). Also, we included an analysis for TVR, all-cause of death and MACE, using the adjusted hazard ratios for some co-variables considered in the observational studies themselves when this information was available.
Results
Characteristics of the trials included
A total of 22 studies were finally included, enrolling 5,543 patients (2,799 patients treated with DES and 2,744 patients treated with BMS)4-8,11-18,23-30. Table 3 shows the main angiographic and clinical baseline characteristics of the trials included.
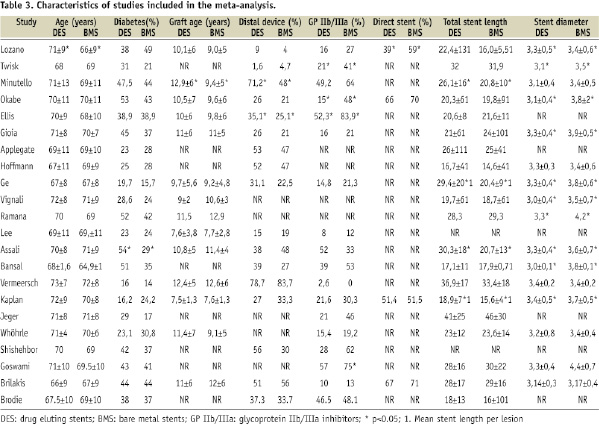
The proportion of diabetic patients ranged from 20% to 54%. Graft age ranged from nine years to 13 years. The use of distal embolic protection device varied from 4 to 84%, and glycoprotein IIb/IIIa inhibitors from 0 to 84%. Stent length ranged from 16 mm to 46 mm, and stent diameter from 3.1 mm to 3.8 mm.
Primary endpoints
EFFICACY ENDPOINT
Data on TVR were available in 4,476 patients (82%) out of 5,543 patients. Figure 2 shows the number of patients who experienced the primary efficacy endpoint of reintervention according to the treatment group, with the OR for each of the studies.
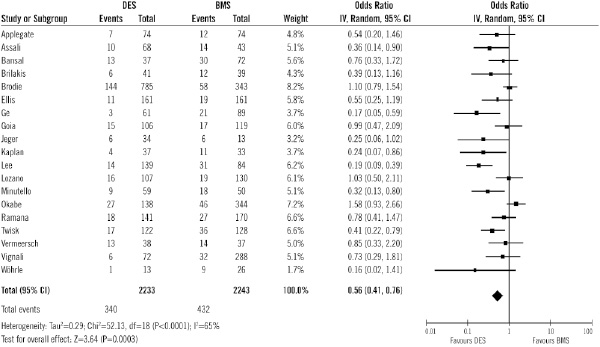
Figure 2. Odds ratios of target vessel revascularisation in patients treated with DES or BMS in each study and in overall patient population. DES: drug-eluting stent; BMS: bare-metal stent
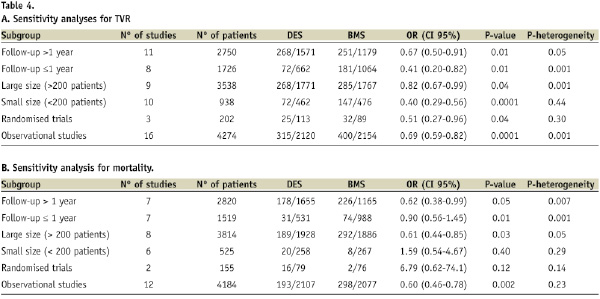
Overall, the use of DES was associated with significant benefits in terms of TVR (15.2% vs. 19.2%; OR of 0.56 [95% CI, 0.41-0.76] p=0.0003), compared with the use of BMS. That means a relative risk decrease of 21% (95% CI 9.5 to 32.4). The number of patients needed to treat with DES to avoid 1 TVR is 25 (16-55). Sensitivity analysis (Table 4) showed that the results were not influenced by the follow-up, sample size or study quality.
Adjusted hazard ratios for TVR were available in five studies, and also TVR was significantly lower in DES compared with BMS (Figure 3).

Figure 3. Adjusted hazard ratios of target vessel revascularisation in each study and in overall population. DES: drug-eluting stent; BMS: bare-metal stent
SAFETY ENDPOINT
Data on all-cause mortality were available in 4,339 (78%) out of the 5,543. Figure 4 shows the number of patients who experienced the primary safety endpoint of mortality according to the treatment group, with the OR for each of the studies.
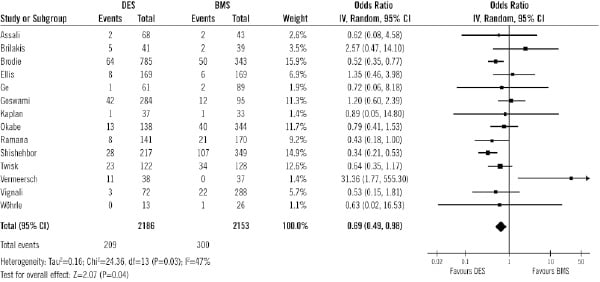
Figure 4. Odds ratios of total mortality in patients treated with DES or BMS in each study and in overall population. DES: drug-eluting stent; BMS: bare-metal stent
Overall, all-cause mortality was significantly lower in DES compared with BMS, (9.5% vs. 13.9%; OR=0.69 [95% CI, 0.49-0.98], p=0.04). That means a relative risk decrease of 31% (95% CI 18 to 45). The number of patients needed to treat with DES to avoid one death is 2316-41. Sensitivity analysis (Table 4) showed differences among subgroups: when studies with more than 200 patients were selected, mortality was significantly lower in the DES subgroup vs. BMS. Also mortality was lower in the subgroup of patients treated with DES vs. BMS when only observational studies were analysed. Adjusted hazard ratios for all-cause death were available in five studies, and also death was significantly lower in DES compared with BMS (Figure 5).
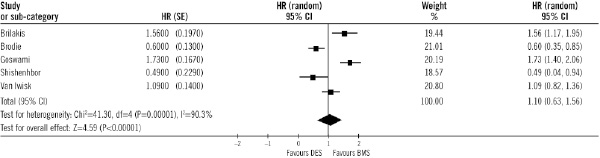
Figure 5. Adjusted hazard ratios of total mortality in each study and in overall population. DES: drug-eluting stent; BMS: bare-metal stent
Secondary endpoints
Data on MACE were available in 4,844 patients (88%) out of 5,543 patients. The rate of MACE was also significantly lower in DES group than in BMS (17.6% vs. 29.2% respectively; OR 0.55, 95% CI 0.42 to 0.71, p<0.0001; Figure 6).
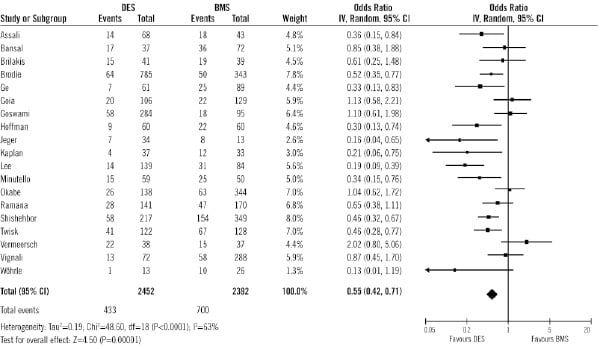
Figure 6. Odds ratios of major adverse cardiac events in patients treated with DES or BMS in each study and in overall patient population. DES: drug-eluting stent; BMS: bare-metal stent
Adjusted hazard ratios for MACE were available in eight studies, and also MACE was significantly lower in DES compared with BMS (Figure 7).
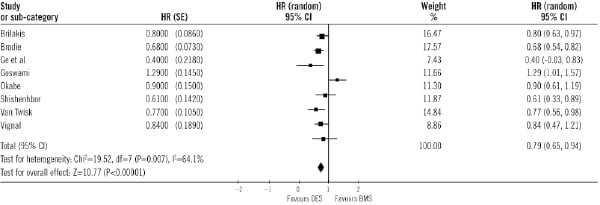
Figure 7. Adjusted hazard ratios of major adverse cardiac events in each study and in overall population. DES: drug-eluting stent; BMS: bare-metal stent
As shown in Figure 8, treatment with DES was associated with a lower cardiac mortality than BMS (6.5% vs. 8.8%; OR of 0.71 [0.51-0.99], p=0.04). That means a relative risk decrease of 25.6% (95% CI 1.2 to 50.1).
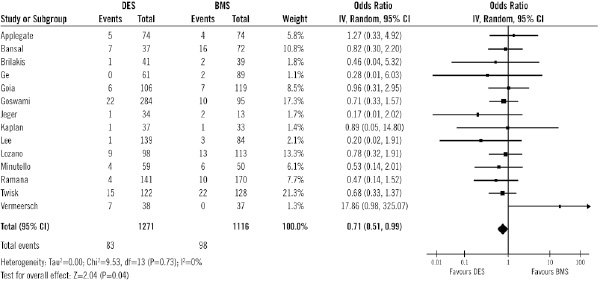
Figure 8. Odds ratios of cardiac mortality in patients treated with DES or BMS in each study and in overall population. DES: drug-eluting stent; BMS: bare-metal stent
Figure 9 shows the absolute numbers of TLR in each treatment group, with the OR for each study. Data on TLR were available in 3,471 patients (79%) out of 4,415 patients.
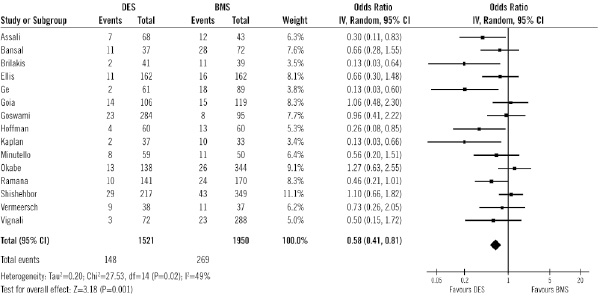
Figure 9. Odds ratios of target lesion revascularisation in patients treated with DES or BMS in each study and in overall patient population. DES: drug-eluting stent; BMS: bare-metal stent
Overall, the use of DES was associated with significant benefits in terms of TLR (9.7% vs. 13.8%; OR of 0.58 [95% CI, 0.41-0.81] p=0.001), compared with the use of BMS.
No difference was observed in the rate of myocardial infarction between DES and BMS (8.3% vs. 7.4%, respectively; OR 0.89, [95% CI 0.60 to 1.32], p=0.57). Also, no difference was found in the rate of stent thrombosis between DES and BMS in the setting of SVGs disease (2.16% vs. 2.35%, respectively; OR of 0.82, [95% CI 0.43 to 1.59)], p=0.56).
Discussion
The main finding of this meta-analysis is that DES implantation is associated with a significant reduction of the need of reintervention of the target vessel in diseased SVGs compared with BMS without an increase in mortality. Moreover, all-cause and cardiac mortality were significantly lower in patients treated with DES. There is also a significant decrease in the need of reinterventions with DES compared to BMS without increment in mortality when studies with follow-up longer than one year are selected.
The percutaneous treatment of degenerated SVGs has been challenging. Use of embolic protection devices and BMS has reduced the procedural complications as distal embolisation, and subsequently long-term cardiovascular events31. Use of DES has decreased the restenotic process caused by neo-intimal hyperplasia, and the rate of repeated revascularisation procedures when treating native coronary arteries. SVGs disease has been poorly represented if not completely excluded in pivotal randomised clinical trials that compared DES versus BMS. Currently, as it has been shown, the comparison of available data of DES versus BMS implantation in SVG disease is limited and based on few retrospective cohort studies, three matched control-case studies and three randomised clinical trials. These randomised clinical trials in the setting of SVGs lesions have several limitations. They included a limited number of patients: 80, 75 and 474,10,27. Two out of the three randomised clinical trials are single-centre and they are a secondary post hoc analysis of the RRISC and BASKET trials10,27. The primary endpoints of RRISC10 and SOS4 trials were angiographic endpoints (12-month binary in-segment restenosis and 6-month in-stent late lumen loss, in SOS and RRISC trials respectively), surrogate markers of the need of reintervention procedures. The primary endpoint in BASKET trial was the cost-effectiveness of DES versus BMS after six months mainly in patients with native coronary artery disease. Therefore, there are no randomised clinical trials designed to assess clinical endpoints at long-term follow-up.
The results of RRISC and SOS trials, the two dedicated randomised trials that evaluate only patients with SVGs lesions, are concordant regarding the primary endpoint. Both trials showed either sirolimus-eluting stents or paclitaxel-eluting stents reduce angiographic restenosis and the need for reintervention compared with bare-metal stents. But, concerns arose from the post hoc analysis of the first randomised clinical trial of sirolimus-eluting stents in the treatment of SVGs, DELAYED RRISC trial13. This showed that the initial benefit of DES on the risk of reintervention was lost at three years suggesting a potential late “catch-up” phenomenon. Also sirolimus-eluting stents were associated with higher mortality at three years possibly related to late or very late stent thrombosis. There is no clear pathophysiological explanation for these findings especially when this phenomenon has not been seen in native coronary arteries and when 3-year mortality rate was surprisingly 0% in patients allocated to BMS and 28% in patients allocated to DES. Interestingly clopidogrel was mandated for only two months in both, DES and BMS. This could have influenced in the high rate of late stent thrombosis in patients allocated to sirolimus-eluting stents. The intravascular ultrasound substudy of RRISC trial32 showed that sirolimus-eluting stents inhibit neointimal hyperplasia compared with BMS in diseased SVGs without evidence of increased incomplete apposition risk, similar to findings in native coronary artery disease.
This meta-analysis showed that DES implantation in diseased SVGs had a significant benefit in terms of reduction in repeated revascularisation procedures without an increase of mortality including all studies with a follow-up longer than one year. Moreover, if only observational studies with a follow-up longer than 30 months are included, DES implantation was associated with a significant decrease in TVR (15.6% vs. 23.2%, OR: 0.63 [95% CI 0.43-0.93; p=0.02]) and a trend toward lower cardiac death (8.3% vs. 12.3%, OR: 0.70 [95% CI 0.47-1.03; p=0.07]). Thus, the finding of late “catch-up” phenomenon was not suggested in observational studies with at least three years of follow-up.
Study limitations
First, the present meta-analysis was not performed on individual patient data. Some caution should be taken into account given the potential clinical heterogeneity among studies, due to definition of variables, study design, and inclusion criteria. Second, DES differ in their structural design, impregnated polymer, type of drug, and pharmacokinetic profile. Therefore, the clinical implication of using different DES in SVGs may be is different. In this meta-analysis, 11 studies included both sirolimus-eluting stents and paclitaxel-eluting stents. Third, most of the BMS used in the published SVG studies were thick-strut. The difference between DES and thin-strut BMS may be smaller than the difference between DES and thick-strut BMS. Forth, currently there is increasing information that prolonged dual antiplatelet regimen may prevent late stent thrombosis. The duration of clopidogrel administration was variable in these studies. Fifth, routine angiographic follow-up may influence the rate of TVR. Both randomised clinical trials, RRISC and SOS, have used routine angiographic follow-up, which may overestimate the need for repeat revascularisation procedures. Sixth, the clinical follow-up was variable among studies. For this reason, a sensitivity analysis was performed according to the clinical follow-up (equal or shorter than one year of follow-up vs. longer than one year). Seventh and the most important limitation is that most of data were derived from retrospective observational series rather than prospective randomised trials. Observational studies have several limitations because of inherent biases and differences in study designs.
Conclusion
In conclusion, this meta-analysis suggested that DES are associated with a significant reduction of the need of reinterventions and mortality at long-term follow-up in diseased SVGs compared with BMS. This is “hypothesis-generating” meta-analysis aimed at pushing funding sources to sponsor adequately sized, prospective, multicentre, and randomised clinical trials to conclusively assess the efficacy and safety of DES in the treatment of VSGs lesions.

