Abstract
Aims: Newer-generation everolimus-eluting stents (EES) have been shown to improve clinical outcomes compared with early-generation sirolimus-eluting (SES) and paclitaxel-eluting stents (PES) in patients undergoing percutaneous coronary intervention (PCI). Whether this benefit is maintained among patients with saphenous vein graft (SVG) disease remains controversial.
Methods and results: We assessed cumulative incidence rates (CIR) per 100 patient years after inverse probability of treatment weighting to compare clinical outcomes. The pre-specified primary endpoint was the composite of cardiac death, myocardial infarction (MI), and target vessel revascularisation (TVR). Out of 12,339 consecutively treated patients, 288 patients (5.7%) underwent PCI of at least one SVG lesion with EES (n=127), SES (n=103) or PES (n=58). Up to four years, CIR of the primary endpoint were 58.7 for EES, 45.2 for SES and 45.6 for PES with similar adjusted risks between groups (EES vs. SES; HR 0.94, 95% CI: 0.55-1.60, EES vs. PES; HR 1.07, 95% CI: 0.60-1.91). Adjusted risks showed no significant differences between stent types for cardiac death, MI and TVR.
Conclusions: Among patients undergoing PCI for SVG lesions, newer-generation EES have similar safety and efficacy to early-generation SES and PES during long-term follow-up to four years.
Abbreviations
ARC: Academic Research Consortium
BMS: bare metal stent(s)
CIR: cumulative incidence rates
DES: drug-eluting stent(s)
EES: everolimus-eluting stent(s)
HR: hazard ratio
IQR: interquartile range
MACE: major adverse cardiac events
MI: myocardial infarction
NSTEMI: non-ST-segment elevation myocardial infarction
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent(s)
SD: standard deviation
SES: sirolimus-eluting stent(s)
ST: stent thrombosis
STEMI: ST-segment elevation myocardial infarction
SVG: saphenous vein graft
TVR: target vessel revascularisation
ULN: upper limit of normal
Introduction
Approximately 3-6% of percutaneous coronary interventions (PCI) are performed among patients with saphenous vein graft (SVG) disease1, and this represents the most important revascularisation option for patients with graft failure. PCI of SVG lesions is characterised by high rates of restenosis and periprocedural myocardial infarction (MI) compared with revascularisation of native coronary arteries. Compared with bare metal stents (BMS), drug-eluting stents (DES) have been shown to reduce the risk of repeat revascularisation by 50%, related to a potent inhibition of neointimal tissue proliferation2 without differences in terms of cardiac death or MI in the largest randomised trial performed to date3,4. However, early-generation DES releasing sirolimus (SES) or paclitaxel (PES) from durable polymers were used in two thirds of patients enrolled in this study1, and little is known regarding the outcomes of newer-generation DES among patients with SVG disease. The newer-generation everolimus-eluting stent (EES) is a thin-strut, cobalt-chromium alloy stent, which is coated with a durable, fluorinated co-polymer releasing a reduced dose of everolimus compared to the dose used with SES5. EES have been shown to improve efficacy and safety compared with early-generation PES6-8 through two years and to provide similar efficacy but improved safety compared with early-generation SES9,10 in a wide range of patients and lesions. However, it is unknown whether the favourable results with the use of newer-generation EES remain sustained among patients undergoing PCI for SVG disease. We therefore investigated the long-term clinical outcomes of patients undergoing PCI of SVG lesions with the use of EES compared with SES and PES in a large-scale registry.
Methods
PATIENT POPULATION
The Bern-Rotterdam registry evaluates clinical outcomes of patients treated with the unrestricted use of DES enrolled at Bern University Hospital, Bern, Switzerland, and the Thoraxcenter, Erasmus Medical Center, Rotterdam, The Netherlands.
Primary results with focus on stent thrombosis have been reported previously6,7,11. In the Dutch institution, SES had been used as a default strategy for PCI as part of the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) registry. From the first quarter of 2003, PES became commercially available and replaced SES as default device and became part of the TAXUS Stent Evaluated At Rotterdam Cardiology Hospital (T-SEARCH) registry. EES (XIENCE V®; Abbott Vascular, Santa Clara, CA, USA, or PROMUS®; Boston Scientific, Natick, MA, USA) had been used as a default strategy for PCI as part of the XIENCE Stent Evaluated At Rotterdam Cardiology Hospital (X-SEARCH) registry since March 1, 2007, until the end of this study period. In the Swiss institution, EES had been used since November 1, 2006, and were implanted on a daily basis alternating with biolimus-eluting stents and zotarolimus-eluting stents. SES had been used since April, 2002, and PES since March, 2003. Individual patients who had been treated with more than one type of DES were excluded from the current registry. The study was approved by the local ethics committee at both institutions and was in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
DATA COLLECTION
All patients were actively followed for major adverse cardiac events using patient administered postal questionnaires including questions on rehospitalisation and major adverse cardiac events. This was complemented by a search of hospital databases at the two institutions. In Bern, the last follow-up took place from February 1, 2007, onwards for patients who had undergone implantation of SES or PES and from February 1, 2010, onwards for patients with EES. In Rotterdam, the last follow-up took place from July 1, 2005, onwards for patients with PES, July 1, 2006, for patients with SES, and April 1, 2010, onwards for patients with EES, respectively. For patients with a suspected event, relevant medical records, discharge letters, and coronary angiography documentation were systematically collected. All suspected clinical events were adjudicated by local cardiologists affiliated with the two institutions, whereas all ST events were adjudicated by an independent clinical events committee whose members were unaware of the type of stent implanted. Baseline clinical and procedural characteristics and all follow-up data were entered into a dedicated database, held at an academic clinical trials unit (CTU Bern, Bern University Hospital, Bern, Switzerland) responsible for central data audits and maintenance of the database.
PROCEDURES
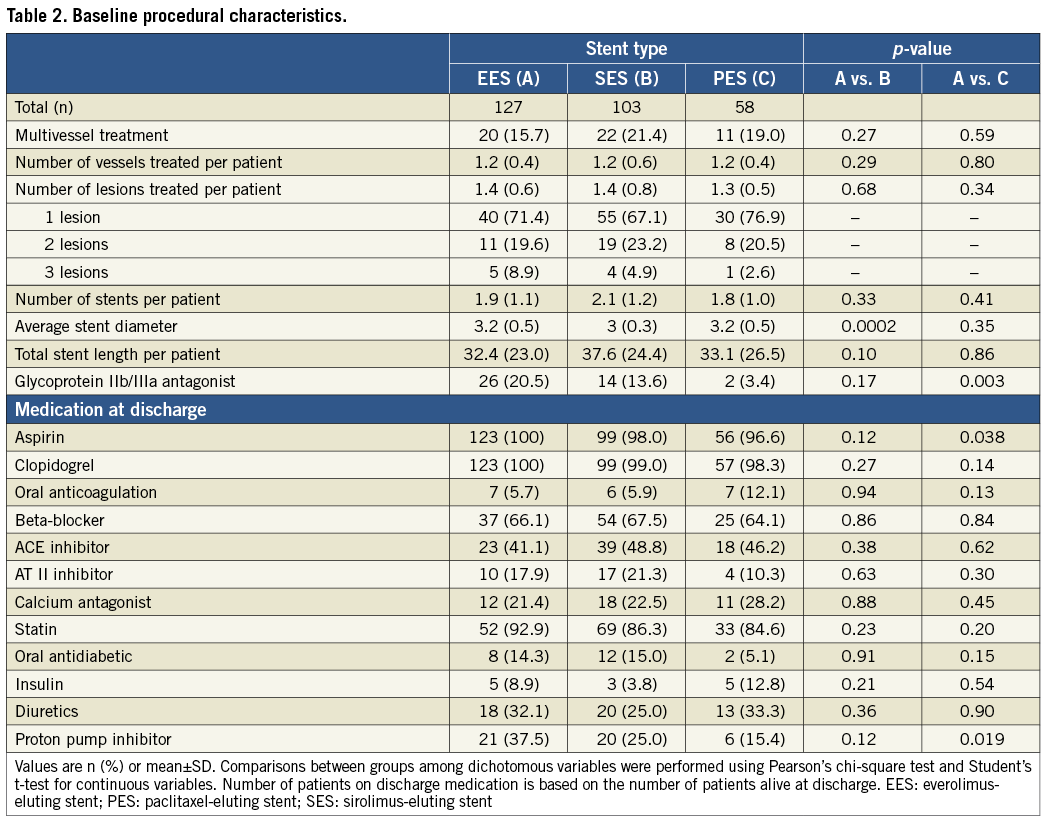
EES were available in diameters from 2.25 to 4.0 mm and in lengths from 8 to 28 mm; SES were available in diameters from 2.25 to 3.5 mm and in lengths from 8 to 33 mm, and PES were available in diameters from 2.25 to 4.0 mm and in lengths from 8 to 32 mm. The procedure and treatment including peri- and post-procedural medication regimen were performed according to current practice guidelines. All patients irrespective of stent type received a loading dose of clopidogrel 300 mg to 600 mg during or immediately after the procedure and were prescribed aspirin once daily lifelong. In the Dutch institution, clopidogrel was administered to patients with SES for at least three months, and for at least six months if patients had received three or more stents, the total stent length was >36 mm, or a chronic total occlusion or bifurcation was treated. Dutch patients treated with EES were prescribed clopidogrel for 12 months. In the Swiss institution, all patients were prescribed clopidogrel for a duration of at least 12 months irrespective of stent type. The use of glycoprotein IIb/IIIa antagonists and distal protection devices was left at the discretion of the operator.
DEFINITIONS
The primary endpoint of this study was major adverse cardiac events (MACE) defined as the composite of cardiac death, MI, and target vessel revascularisation up to four years. The definition of cardiac death included any death due to immediate cardiac cause, procedure-related deaths, unwitnessed death and death of unknown cause. The diagnosis of MI was based on an elevation in CK to more than twice the upper limit of normal (ULN) and an elevation of CK-MB to more than three times ULN in the presence of ischaemic symptoms or ischaemic ECG changes. Target vessel revascularisation (TVR) was defined as any repeat percutaneous intervention or surgical bypass of any segment within the entire major coronary vessel proximal and distal to the target lesion, including upstream and downstream branches and the target lesion itself. Target lesion revascularisation (TLR) was defined as a repeated revascularisation due to a stenosis within the stent or within the 5 mm borders proximal or distal to the stent. A 12-lead electrocardiogram was obtained prior to the procedure and within 24 hours after PCI. Additional ECGs were obtained in case of recurrent signs or symptoms of ischaemia. Acute coronary syndrome was defined as acute myocardial ischaemia based on clinical symptoms, electrocardiographic changes, and elevation of cardiac biomarkers, and encompassed an acute ST-segment (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina. Definitions of hypertension, hyperlipidaemia and renal dysfunction were reported previously7,11. Stent thrombosis was defined according to the Academic Research Consortium (ARC)8,9.
STATISTICAL ANALYSIS
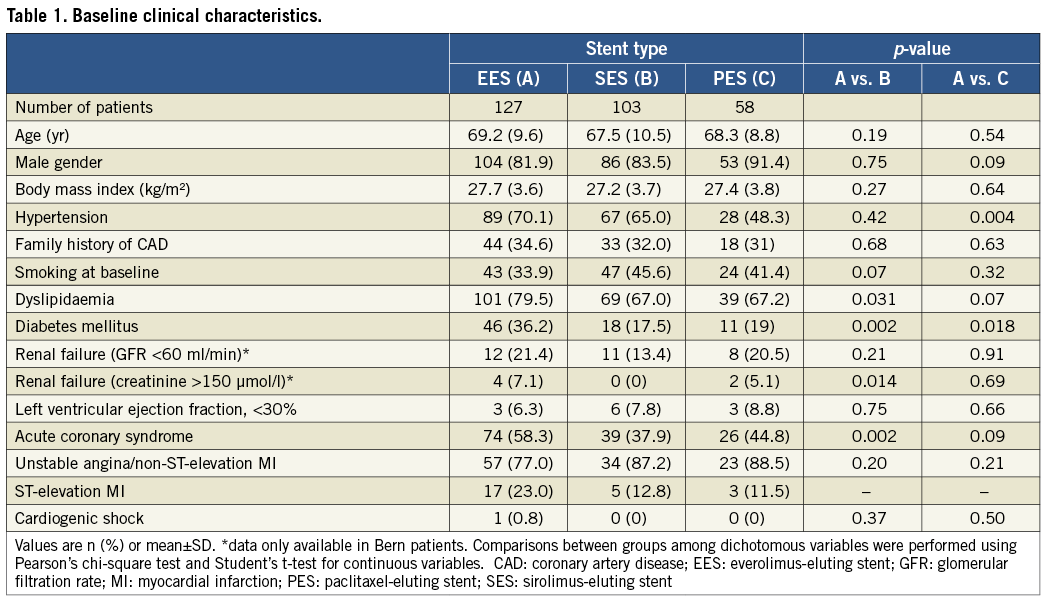
Baseline clinical and procedural characteristics of the three stent types are presented as counts and percentages for dichotomous variables and as mean and standard deviation (SD) for continuous variables. Pearson’s chi-square test and Student’s t-test were used for comparing dichotomous and continuous variables, respectively. Cumulative incidence rates (CIR) per 100 patient years were calculated for each endpoint, defined as the number of new events occurring during a specific time period divided by the total number of patient years observed. In contrast to crude percentages, CIR take into account differences in follow-up duration between different stent types. Propensity scores for receiving EES were estimated for each centre by the use of a logit model including age, gender and pre-treatment variables associated with stent selection at p<0.10 (i.e., family history of coronary artery disease, acute coronary syndrome and cardiogenic shock for both centres; body mass index and left ventricular ejection fraction as additional variables for Bern; arterial hypertension, smoking, diabetes and hyperlipidaemia as additional variables for Rotterdam). Propensity scores were used to derive inverse probability of treatment weights, with the inverse of propensity score as analytical weights in EES-treated patients and the inverse of 1 minus the propensity score among early-generation DES-treated patients. Comparisons between stent types were performed with a Cox proportional hazards model, crude and adjusted using inverse probability of treatment weighting. All statistical analyses were performed using STATA release 11.1 (StataCorp, College Station, TX, USA). All p-values are two-sided.
Results
Between April 16, 2002, and March 31, 2009, 12,339 consecutive patients underwent treatment with the unrestricted use of EES (n=4,212), SES (n=3,819) and PES (n=4,308). Out of this cohort, 288 patients (5.7%) (177 [61.5%] enrolled at Bern University Hospital, and 111 [38.5%] included at Thoraxcenter, Rotterdam) underwent PCI of at least one SVG lesion with the use of EES among 127 patients, SES among 103 patients, and PES among 58 patients. Baseline clinical characteristics for all three stent types are summarised in Table 1. Patients treated with EES compared with those treated with either SES or PES more frequently had diabetes. Patients treated with EES were more frequently hypertensive compared to those treated with PES, and more frequently had dyslipidaemia, renal failure and presented with an acute coronary syndrome than SES-treated patients. Table 2 shows procedural characteristics, which were balanced among the three treatment groups with the exception of a larger stent diameter in lesions treated with EES compared with those treated with SES. The use of glycoprotein IIb/IIIa antagonists, aspirin, and proton pump inhibitors was more frequent among EES compared with PES-treated patients.
Clinical outcome
The median follow-up duration among surviving patients completing the last follow-up was 2.5 years in patients treated with EES (interquartile range: IQR 1.9 to 3.2 years), four years in patients treated with SES (IQR 3.0 to 4.0 years), and 3.5 years in patients treated with PES (IQR 2.3 to 4.0 years) with an accumulated 144, 266, and 302 patient years, respectively.
Clinical outcomes up to four years are summarised in Table 3 and Table 4.
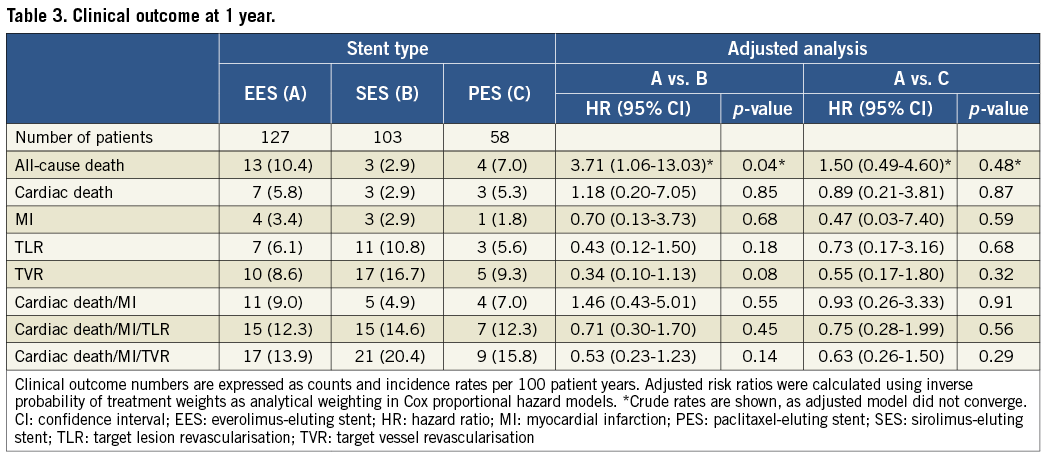
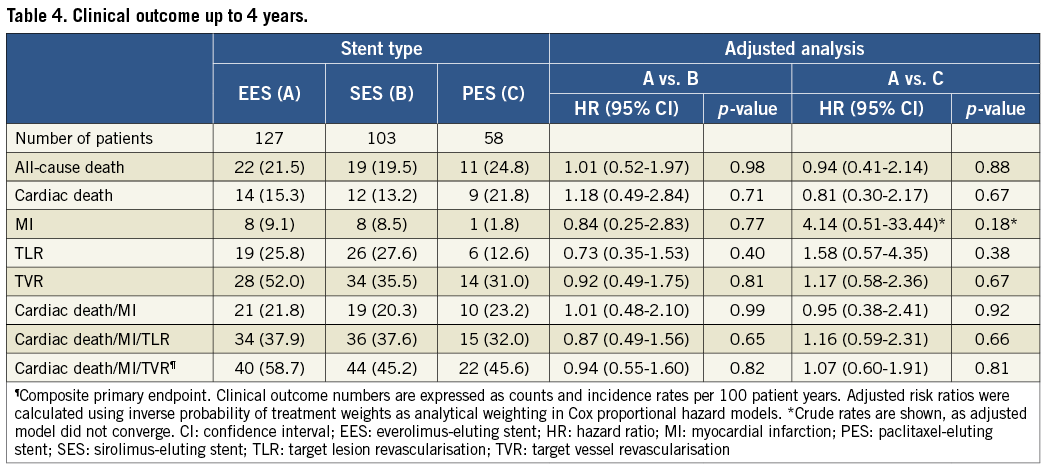
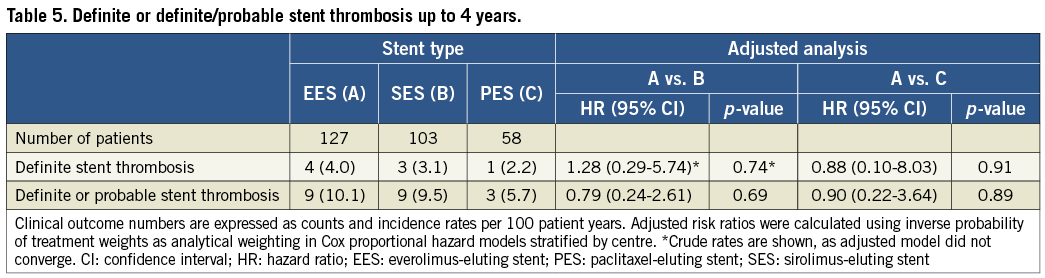
Up to four years, incidence rates per 100 patient years for the primary endpoint MACE were similar among patients treated with EES (58.7%), SES (45.2%, adjusted HR 0.94, 95% CI: 0.55-1.60) and PES (45.6%, adjusted HR 1.07, 95% CI: 0.60.-1.91) in adjusted analyses (Table 3 and Table 4, Figure 1). Similarly, there was no difference in the risk of cardiac death (EES vs. SES adjusted HR 1.18, 95% CI: 0.49-2.84, EES vs. PES adjusted HR 0.81, 95% CI: 0.30-2.17), MI (EES vs. SES adjusted HR 0.84, 95% CI: 0.25-2.83, EES vs. PES crude HR 4.14, 95% CI: 0.51-33.44), and TVR (EES vs. SES adjusted HR 0.92, 95% CI: 0.49-1.75, EES vs. PES adjusted HR 1.17, 95% CI: 0.58-2.36) in adjusted analyses. The incidence rates per 100 patient years for definite ST and definite or probable ST showed no differences among stent types at any time point (Table 5).
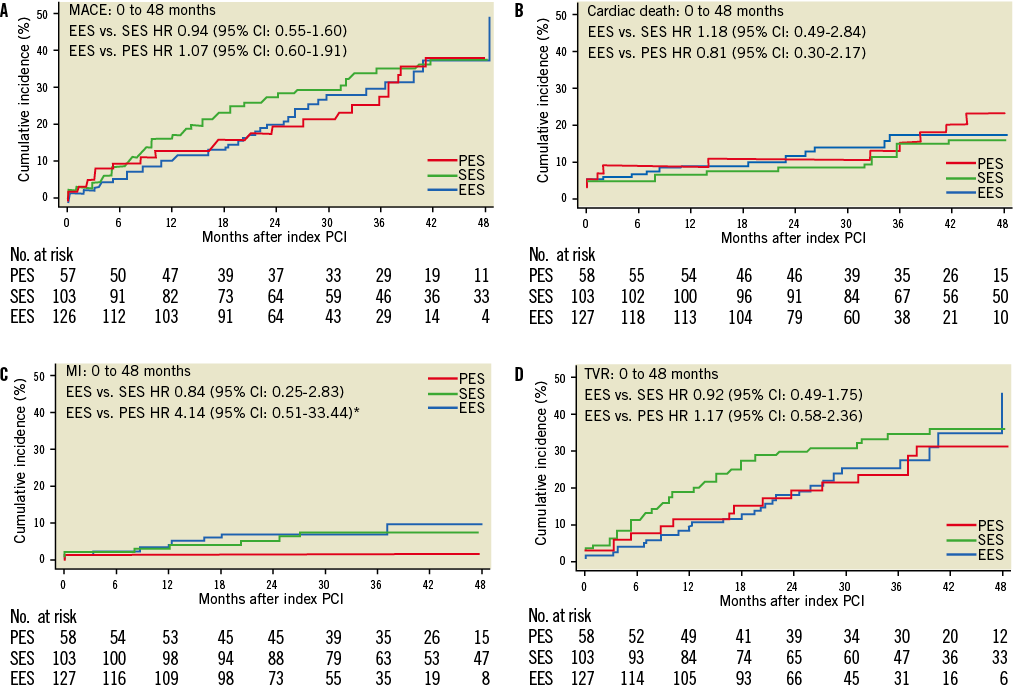
Figure 1. Cumulative event curves for the primary endpoint of major adverse cardiac events (MACE) (A), cardiac death (B), myocardial infarction (MI) (C), and target vessel revascularisation (TVR) (D) up to 48 months. *Crude hazard ratio is shown, as adjusted model did not converge. EES: everolimus-eluting stents; PES: paclitaxel-eluting stents; SES: sirolimus-eluting stents
The duration of dual antiplatelet therapy differed between the two institutions. In order to analyse potential site-specific differences in outcomes comparing EES with early-generation DES, we performed a sensitivity analysis for the primary outcome and found hazards to be similar for both institutions regarding the primary endpoint (Bern EES vs. early-generation DES: HR 0.94, 95% CI: 0.55-1.60, p=0.82; Rotterdam EES vs. early-generation DES: HR 1.07, 95% CI: 0.60-1.01, p=0.82).
Discussion
This is the first report comparing newer-generation EES with early-generation SES and PES during long-term follow-up among patients undergoing PCI for SVG disease. The main findings of our study are: 1) the use of EES resulted in similar safety and efficacy compared to the use of early-generation SES and PES among patients with SVG lesions; 2) event rates for restenosis and recurrent ischaemia were exceedingly high during follow-up through four years regardless of the type of DES implanted.
Limited data are available on the treatment of SVG lesions with coronary artery stents.
A comparison of DES with BMS in SVG lesions in a total of 5,543 patients followed for at least one year yielded similar results to those observed in other patient populations, namely a substantial improvement in the need for repeat revascularisation of the target vessel without differences in terms of MI or stent thrombosis. Differences in cardiac death were not recorded when taking into account only randomised trials12. However, conflicting results were observed among the few studies investigating outcomes beyond one year. The randomised Extended Duration of the Reduction of Restenosis In Saphenous vein grafts with Cypher stent (DELAYED RRISC) study suggested an increased risk of cardiac death and numerically lower rates of MI with SES compared with BMS as well as a loss of the initially observed lower risk of TVR during long-term follow-up. Conversely, the long-term results of the Stenting of Saphenous Vein Grafts (SOS) trial suggested a similar risk of cardiac death but a lower risk of MI as well as sustained efficacy in terms of repeat revascularisation among PES compared with BMS-treated patients with SVG disease during long-term follow-up.
Newer-generation DES have been designed to improve upon the limitations of early-generation DES by reducing stent strut thickness, increasing the biocompatibility of polymers and modifying drug content. Several randomised clinical trials as well as large-scale registries confirmed improved safety and efficacy of newer-generation EES compared with PES and SES in a wide range of patient and lesion subsets. To date, only one study has compared early-generation DES with a newer-generation stent releasing sirolimus from a biodegradable polymer among patients undergoing treatment of SVG lesions and observed no difference in terms of the primary endpoint including cardiac death, MI, and repeat revascularisation13. As it relates to long-term results, no data are available at this point in time.
Our study is the first to compare newer-generation EES with early-generation SES and PES among patients undergoing PCI of SVG lesions during long-term follow-up through four years, and is of particular interest due to the unselected, consecutive patient population undergoing PCI with the unrestricted use of DES. Similar to outcomes in ISAR-CABG, outcomes for the primary endpoint and its individual components were similar for newer-generation DES compared with early-generation SES and PES. Even when considering device-specific endpoints such as cardiac death, MI and TLR as well as stent thrombosis, no differences were noted among these devices throughout the entire follow-up period.
Irrespective of stent type, adverse events were much more frequent among patients undergoing PCI of SVG lesions compared to those undergoing PCI of native coronary arteries. Specifically, rates of MACE at four years in the present study (46%) were similar to those reported among PES-treated patients in the randomised Stenting of Saphenous Vein Grafts (SOS) trial at 35 months of follow-up (54%)14. Similarly, in the Extended Duration of the Reduction of Restenosis In Saphenous vein grafts with Cypher stent (DELAYED RRISC) study15, rates of MACE amounted to 58% among SES-treated patients at a median follow-up of 32 months. These figures contrast with rates of MACE in the range of 20% among unselected patients enrolled in all-comers studies with the predominant treatment of native coronary artery lesions16-18. Of note, clinical outcomes were driven by high rates of death, restenosis of the target lesion as well as disease progression within the target vessel, reflecting the advanced stage of coronary artery disease in this patient population.
Potential explanations for the lack of benefit with newer-generation EES compared with early-generation DES in the specific subset of SVG lesions may have been the small patient population. However, considering the high event rates and the long-term follow-up, hazards would be expected to favour EES, assuming similar benefits in terms of relative risk reduction observed in pivotal trials and all-comer patient populations. Differences between SVG lesions and native coronary arteries in terms of periprocedural treatment characteristics, atherosclerotic disease burden as well as the interaction with revascularisation by means of drug-eluting stents may be of relevance. Brilakis and colleagues reported an increased risk for in-hospital mortality among patients undergoing PCI for treatment of SVG compared to native coronary artery lesions (HR 1.22, 95% CI: 1.12-1.32, p<0.001). This was related to differences in patient and lesion risk profile and a higher incidence of acute complications such as no reflow19. SVG failure remote from the stented lesion (TVR without TLR) occurs in 30-50% of all repeat revascularisation procedures. This proportion is certainly higher compared with lesions involving native coronary arteries20,21, suggesting that non-stented disease progression remains an important adverse event among patients with SVG disease. Although rates of target lesion revascularisation during the first year in the present study were very much comparable to those observed following treatment of native coronary artery lesions, recurrent ischaemia related to the stented segment became increasingly apparent at a later time, suggesting a considerable lack of long-term efficacy. Specifically, annual rates of TLR between the first and fourth year of follow-up were 50 to 70% higher compared with annual rates previously reported in the context of native coronary artery disease16. Therefore, SVG lesions continue to represent an important lesion subset with inadequate efficacy following the use of newer-generation DES.
Pathological analyses and experimental animal models have contributed to our understanding of accelerated atherosclerosis in SVG lesions22. Mechanical stress induced by a substantial change in haemodynamics from a venous to an arterial circulation has been identified as an important source of saphenous vein graft wall thickening, largely related to gene expression of adhesion molecules, which evoke inflammatory processes and signal pathways resulting in proliferative cell growth. Neointimal formation is followed by macrophage infiltration and eventually necrotic core formation, resulting in vulnerable plaque formation. Stent implantation of SVG lesions more often lead to strut penetration into the necrotic core, which may delay healing and perpetuate inflammation, compared with stents implanted into native coronary artery lesions resulting in an increased risk for thrombotic occlusions23. In addition, neoatherosclerotic changes have been observed as early as one year after stent implantation in SVG lesions, which is more premature than observed in native coronary artery lesions. Although the prevalence of neoatherosclerosis within DES-treated SVG lesions has not been assessed to date, pathology studies suggest that neoatherosclerosis is an important mechanism contributing to restenosis during long-term follow-up, providing a potential explanation for the high TLR rates observed in this study beyond one year.
Very late stent thrombosis is one of the major concerns with the use of early-generation DES; however, the use of EES was associated with a substantial reduction in an all-comers patient population9,10. In the present study, there were no differences among the three stent platforms. However, event rates and patient population were small, precluding further exploration of differences among devices.
Limitations
The present study has to be interpreted in view of the following limitations. First, this study was not specially designed to compare the safety and efficacy of newer-generation EES with early-generation DES in SVG lesions. The data are derived from a non-randomised, observational cohort. Second, we lack information regarding the diameter of SVG lesions, the use of distal protection devices, and the age of SVGs at the time point of the intervention. Third, patients were enrolled during different time periods and advances in interventional techniques (e.g., more frequent post-dilatation) may have impacted on results. In addition, the follow-up period differed among the three treatment groups. However, we employed statistical methodologies to present adjusted analyses by employing inverse probability of treatment weights and the reporting of cumulative incidence rates. Finally, the sample size of this study is small; larger patient populations are needed to address more definitively the value of newer-generation DES in SVG lesions.
Conclusions
Among patients undergoing PCI for SVG lesions, newer-generation EES provide similar safety and efficacy compared to early-generation SES and PES during long-term follow-up. The high rates of adverse events among patients with SVG disease are related to disease progression of treated and untreated SVG segments.
Guest Editor
This paper was Guest Edited by Andreas Baumbach, MD; Bristol Heart Institute, University Hospitals Bristol, Bristol, United Kingdom
Conflict of interest statement
M. Taniwaki is the recipient of a research grant from Abbott and OrbusNeich. S. Windecker has received research grants from Abbott, Boston Scientific, Biosensors, Cordis, Medtronic and St. Jude. B. Meier has received research grants from Abbott and Cordis. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

