Abstract
Aims: Treatment of saphenous vein graft (SVG) disease is still a matter of debate given the uncertainty of the available conflicting data. Our aim was to assess, by means of a meta-analytic approach, the risk/benefit profile of drug eluting stents (DES) versus bare metal stents (BMS) in the treatment of SVG disease.
Methods and results: A search of relevant studies in several databases was performed. The endpoints of interest such as: major adverse events (MAE) (the combination of overall death and non-fatal myocardial infarction [AMI]), target vessel revascularisation (TVR), and target lesion revascularisation (TLR) have been calculated in-hospital and at the longest follow-up. Single endpoints and the rate of stent thrombosis (ST) were also assessed. Three randomised controlled trials and 15 registry studies were appraised, totalling 3,294 patients. During hospitalisation, there was no difference in the risk of MAE, overall death, AMI and TVR. No data were available to calculate the TLR rate. At a mean follow-up of 19.8 months, no significant differences were found in the risk of MAE and AMI. BMS were associated with a trend towards a higher risk of overall death (OR 1.32 [1,00-1.74], p=0.05, number needed to treat [NNT]=55). DES showed superiority in terms of TVR (OR 1.86 [1.33-2.61], p=0.0003, NNT=16), and TLR (OR 1.77 [1.27-2.48], p<0.0001, NNT=25). According to pre-specified subgroup analyses, these effects seem less evident at the long-term follow-up. DES were not associated with an increased risk of ST.
Conclusions: Use of DES in SVG substantially reduces both TVR and TLR. These data also demonstrate that using DES in SVG is safe and contradict previous reports of potential risks.
Introduction
Coronary artery bypass grafting surgery (CABG) dramatically changed the management of patients with ischaemic heart disease as it proved to improve symptoms and life expectancy in particular in those patients with multivessel disease. Despite the superiority of arterial over venous conduits, saphenous vein grafts (SVG) are still the most commonly used1 even though they have a rate of long-term failure of almost 50% at 10 years2. Significant atherosclerotic disease of SVG, despite optimal medical therapy, may result in the recurrence of angina and a higher risk of major adverse events1. The treatment of patients with evidence of SVG failure is still a matter of debate for both interventional cardiologists and cardiac surgeons. Given the high risk of further surgical treatment3 and the availability of new technologies including protection devices and ad hoc catheters, percutaneous coronary intervention (PCI) is currently the preferred approach4,5.
The superiority of bare metal stents (BMS) over balloon angioplasty is widely accepted even in this particular setting6. On the other hand, the encouraging data about the beneficial effect of drug eluting stents (DES) over BMS in terms of the risk of restenosis and repeat revascularisation when deployed in native coronary arteries acted also as a spur for the utilisation of such devices for “off label” indications like SVG disease7. Notably, along with several study registries reporting disparate results, there is very little data concerning a randomised comparison of BMS vs. DES in SVG treatment8-11. Of note, data from the RRISC trial, while showing a consistent superiority of DES in the short-term follow-up8, reported a puzzling increase of death and stent thrombosis in the DES arm at the three year follow-up9 which was not confirmed by the SOS trial10 and the recently published SVG subgroup analysis of the BASKET trial11. Thus, a general consensus on this topic is still lacking. By means of a meta-analytic approach, we aimed at summarising the current evidence in order to provide thoughtful insights in such an unclear scenario.
Methods
Study selection
BioMedCentral and PubMed were searched without language restrictions (updated to March 2009), according to an established method using as key words “saphenous vein graft” and “stent”(see Appendix)12. Pertinent studies were also searched in major recent international cardiology meetings. References of original and review articles were cross-checked.
Data extraction and endpoints of interest
Two trained and independent reviewers (TL, AP) performed data abstraction blindly. Divergences were resolved by consensus or by a third reviewer. The endpoints of interest were the combined rate of major adverse events (MAE), defined as the cumulative risk of all cause death and nonfatal acute myocardial infarction, target vessel revascularisation (TVR) and target lesion revascularisation (TLR). Additional analyses were carried out according to single endpoints and the rate of stent thrombosis. Endpoint assessment was performed both for in-hospital and longest follow-up available.
Primary analyses included all the available studies. Secondary analyses were performed by subgrouping studies according to follow-up duration of six months, 6 to 12, 12 to 24 and more than 24 months. Separate analyses were also performed pooling data from registries and randomised controlled trials.
Moreover, we assessed whether there are distinctive effects of sirolimus- (SES) and paclitaxel- (PES) eluting stents compared to BMS by subgrouping studies according to the type of stent used.
Data synthesis and analysis
Review Manager 4.2.513 was used. Review Manager is a comprehensive statistical and reviewing program, developed and maintained by The Cochrane Collaboration, which includes ad hoc statistical tools for pooled estimate calculations, according to several methods.
Statistical analysis
Odds ratios with 95% confidence intervals (95%CI) were used as summary statistics. Binary outcomes from individual studies were combined with both Der Simonian and Laird random-effect model and fixed-effect model, according to an intention to treat analysis. We also carried out the “z” test where z=estimated effect size / standard error of the estimated effect size, and the odds ratio considered on the log scale. As log (OR) has a unimodal distribution, the reported “z” values were analysed to obtain a two-tailed “p”, and hypothesis testing results were considered statistically significant at the 0.05 level14. We also calculated the number needed to treat (NNT) to prevent a MAE as the inverse of absolute risk reduction (ARR): NNT=1/ARR.
We computed Cochrane Q heterogeneity test (H) by summing the squared deviations of each study’s estimate from the overall meta-analytic estimate, weighting each study’s contribution in the same manner14. We used the Q together with the resulting degrees of freedom (df) to calculate the proportion of variation due to heterogeneity (Inconsistency: [I2]=[Q-df]/Q). The degree of inconsistency among studies (I2) was estimated with scores of <25%, between 25% and 75%, and >75% representing, respectively, low, moderate or high inconsistency15.
Sensitivity analysis was performed by excluding trials one at a time in order to assess the contribution of each study to the pooled estimates14.
The likelihood of publication bias was assessed graphically by generating a funnel plot for the combined endpoint of MAE and mathematically by means of Egger’s test (p for significant asymmetry <0.1)16.
This study is inspired by the good practice guidelines17, including those from the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group18 and the Cochrane collaboration Newcastle-Ottawa scale for assessing quality of cohort study14.
Results
Search result
The search algorithm resulted in 220 citations. We eventually appraised 18 studies, three randomised controlled trials8-11 and 15 registries19-33 totalling 3,294 patients with a mean follow-up of 19.8 months (range 6-48) (Figure 1).
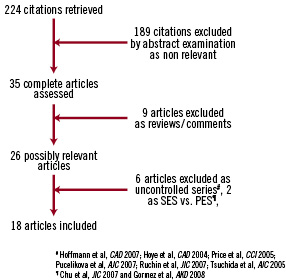
Figure 1. Flow diagram according to QUOROM statement. PES: paclitaxel eluting stent; SES: sirolimus eluting stent
Main characteristics of included studies are shown in the Table 1.
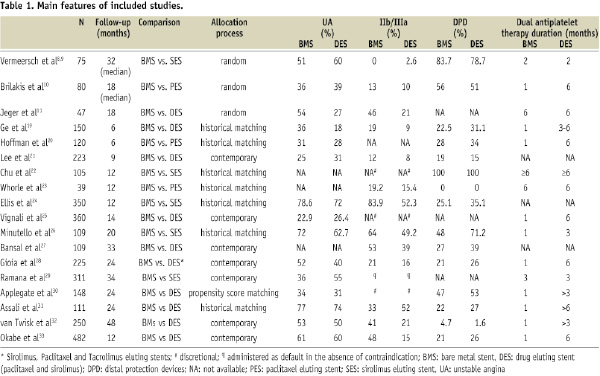
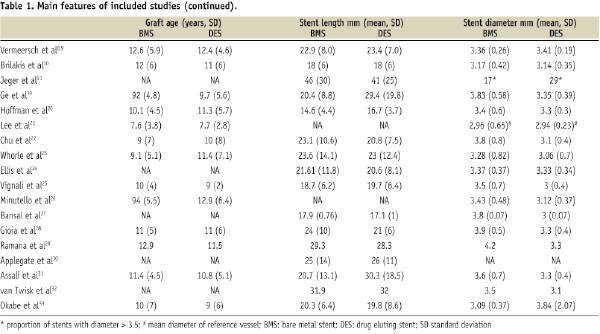
Five studies compared BMS versus sirolimus eluting stents (SES)8,9,22,24,26,29, three BMS versus paclitaxel eluting stents (PES)10,20,23, nine BMS versus both PES and SES11,19,21,25,27,30-33. Only one study included also tacrolimus eluting stents28. Baseline characteristics of the enrolled patients and the incidence of the common cardiovascular risk factors were similar across studies. The percentage of patients admitted with ST-elevation myocardial infarction was reported only in five studies where it ranged between 2% and 11%11,20,24,29,31,33. The relative percentage of patients with stable and unstable angina was highly variable across studies as well as the use of distal protection devices and IIb/IIIa inhibitors. Definitions of endpoints were fairly homogeneous thus allowing the pooling of dichotomous data.
In-hospital endpoints
Few studies reported in-hospital endpoints. Pooling data with either “random-effect” or “fixed-effect” model yielded similar results, which are shown according to the former. There was no difference in the risk of MAE (0.90 [0.49-1.67], p=0.74, p for H=0.44, I2=0%) as well of overall death (0.98 [0.22-4.47], p=0.98, p for H=0.45, I2=0%), AMI (0.97 [0.57-1.67], p=0.92, p for H=0.52, I2 0%) and TVR (3.70 [0.66-20.72], p=0.14, p for H=0.83, I2=0%) between patients treated with DES vs. BMS. No data were available to calculate TLR rate.
Long-term follow-up endpoints
Pooling raw data according to “random-effect” model yielded different results with respect to the “fixed-effect” model. Because of the presence of heterogeneity and moderate inconsistency, results are shown and discussed according to the former.
No significant differences were found according to the risk of MAE (1.25 [0.89-1.67], p=0.19, p for H=0.006, I2 52%) and AMI (1.15 [0.69-1.92], p=0.6, p for H=0.02, I2 47%) while there was a trend towards a beneficial effect of DES in terms of overall death (1.32 [1,00-1.74], p=0.05, p for H=0.44, I2 0% ARR=0.02, NNT=50 [25-90]) (Figure 2).
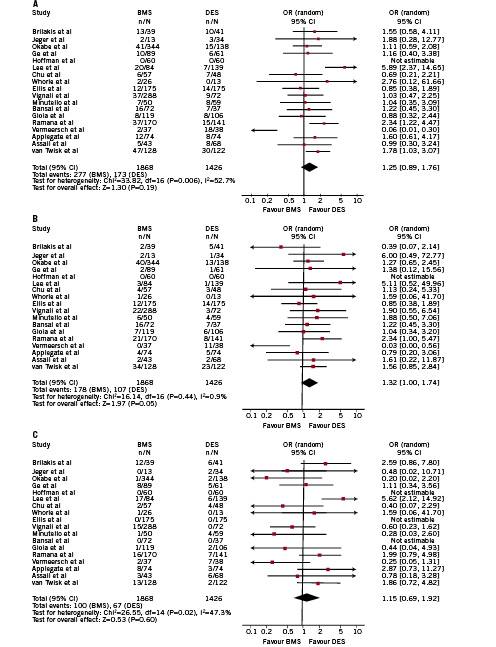
Figure 2. Overall analysis of the risk of (A) major adverse events (MAE), (B) overall death and (C) acute myocardial infarction (AMI) at the longest follow-up available. Single study odd ratios and 95% confidence intervals are shown by squares and lines. Overall odd ratio with 95% confidence interval shown by diamonds.
DES showed superiority in terms of a reduced risk of TVR (OR 1.86 [1.33-2.61], p=0.0003, p for H=0.0009, I2 59%, ARR=0.06, NNT=16 [7-25]), and TLR (OR 1.77 [1.27-2.48], p<0.0001, p for H=0.13, I2 0%, ARR=0.04, NNT=25 [10-100]) (Figure 3).
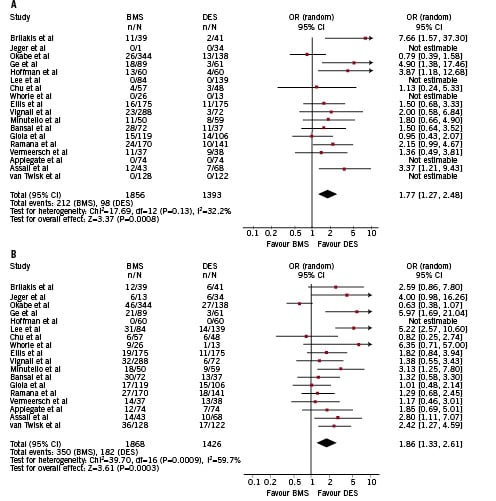
Figure 3. Overall analysis of the risk of (A) target lesion revascularisation (TLR) and (B) target vessel revascularisation (TVR) at the longest follow-up available. Single study odd ratios and 95% confidence intervals are shown by squares and lines. Overall odd ratio with 95% confidence interval shown by diamonds. ARR: absolute risk reduction; NNT: number needed to treat
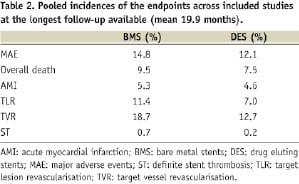
Absolute incidences of endpoints across the included studies are shown in Table 2. Importantly, the risk of stent thrombosis was not different between DES and BMS (OR 1.86 [0.52-6.61], p=0.34, p for H=0.26, I2 24%).
Pre-specified subgroup analyses
In the subgroup of studies with follow-up duration of six months, no differences were observed for the risk of MAE, AMI and overall death. On the other hand, DES showed large superiority in terms of TVR (5.97 [1.69-21.04], p=0.0005, ARR=0.1, NNT=10 [7-25]) and TLR (4.32 [1.82-10.28], p=0.0009, ARR 0.14, NNT=7 [4-14]).
In the subgroups of studies with follow up duration of 6-12 months, as well as 12-24 months, no differences were found with respect to all the endpoints.
In the subgroup of studies with follow-up duration of >24 months, DES were slightly significantly associated with a reduction of TVR (1.62 [1.16-2.25], p=0.04, ARR=0.07, NNT=15 [7-50]). No differences were found for other endpoints.
Sensitivity analysis
We performed explorative analyses initially excluding one study at a time and then pooling the randomised controlled trials and the registries separately. The exclusion of one study at a time did not significantly alter the pooled ORs. Of note, the pooled analysis of the randomised controlled trials showed superiority of the DES over BMS only in terms of TVR at the longest follow-up with an OR of 1.95 (1.04, 3.67), p=0.04, ARR 0.14, NNT 74-15.
On the other hand, in the pooled analyses of the registries, DES were associated with a significant benefit in terms of MACE (1.45 [1.15-1.81], p=0.001, p for H=0.14, ARR 0.03, NNT 33 [19-60]), overall death (1.4[1.06, 1.85], p=0.02, p for H=0.95, ARR 0.03, NNT 33 [22-55]), TVR (1.7 [1.38, 2.10], p<0.001, p for H=0.0004, ARR 0.07, NNT 14 [7-34]), and TLR (1.67[1.27, 2.20], p=0.0003, p for H=0.18, ARR 0.04, NNT 25 [16-48]) while showing only a trend in favour of DES for the risk of AMI. When comparing raw data from historical matched registries no difference in terms of MACE, overall death and AMI was found, while DES showed superiority over BMS in terms of reduced TVR (OR 2.48 [1.49-4.13], p=0.003) and TLR (2.3 [1.48-3.55], p= 0.02). On the other hand, when pooling raw data from “contemporary” registries DES were associated with a reduced risk of MACE (OR 1.64[1.07-2.51], p=0.02) and death (OR 1.54 [1.10-2.14], p=0.03), while no difference was found in terms of AMI, TLR and TVR.
Three studies specifically used PES10,20,23 while 5 SES 9,22,24,26,29. Overall consistency was found without apparent difference between different stents and the overall analyses.
Quality of included studies and assessment of possible biases
Very good overall consistency has to be acknowledged among reviewers rating the quality of the studies (LT, PA). As for the study registries, discrepancies in the design of the study (Table 1), the lack of a “sequence generation” for the “allocation process”, which was not “concealed”, along with the lack of “blinding” might have introduced possible biases.
Nevertheless, included patients were well representative of the “real-world” scenario according to the incidence of risk factors and baseline characteristics. The presence of “incomplete data”, whereas applicable, has been thoughtfully addressed, and no “selective reporting” has to be acknowledged.
Overall the quality of the registries has to be acknowledged as poor (i.e., high likelihood of biases) while the randomised controlled trials had a good internal validity (i.e., low likelihood of biases).
The Funnel plot for all studies according to the risk of MAE, death, AMI, TLR and TVR (Figure 4) showed an overall symmetry within the 95% confidence interval. Moreover, Egger’s test for the risk of MAE further confirmed the absence of small study/publication bias as “p for asymmetry” was 0.13.
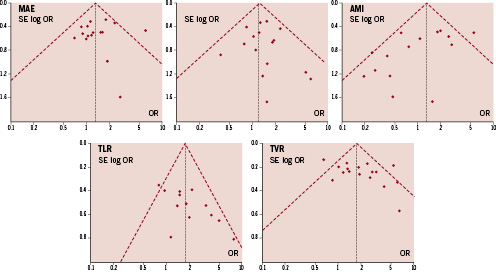
Figure 4. Funnel plots of included studies according to the endpoints. Dotted lines represent 95% confidence interval.
Discussion
The result of this pooled analysis of all the available studies showed that the use of DES in the setting of SVG treatment does not increase the risk of death, infarctions and stent thrombosis, conversely to previous reports seriously addressing the safety of DES in SVG. Drug eluting stents appear to be beneficial compared to BMS by significantly reducing TVR and TLR.
According to this analysis, treating 100 patients with SVG disease with a DES would prevent, at a mean follow-up of 19.8 months, 10 TVR and four TLR compared to BMS. Of note, such advantage seems mostly driven by the beneficial effect of DES on short-term follow-up, while at longer term the advantage seems much less evident if not absent at all.
The treatment of SVG disease represents about 5-10% of the cases of the catheterisation laboratory4. Atherosclerotic process in SVG has several peculiarities which account for the poor outcome compared to coronary arteries. Lesions in SVG are generally associated with a higher plaque burden, more friable material and frequent superimposed thrombosis which conceivably relate to the higher risk of distal embolisation and periprocedural myocardial damage34,35. Plaque composition is also peculiar as it is lipid-richer, softer and, as a consequence, more prone to rupture36.
As soon as the technology of BMS had been introduced, its advantage over balloon angioplasty was clear6, however the outcome of BMS in SVG was still poor when compared to interventions in native coronaries as the rate of 30-days MACE was about 10% while the rate of 6-months restenosis was >30%4. These findings led to several studies investigating the in-stent restenosis phenomenon in SVG and eventually to the clarification that even the restenotic process was different as it was based on several distinct phenomena including intimal hyperplasia, progression of atherosclerosis, local inflammatory reaction and thrombosis37 whilst the major process in the coronaries is intimal hyperplasia38. On the other hand, following their consistently positive results when implanted in native arteries39-41, DES have been thought able to overcome the limitations of BMS. Three randomised controlled trial have been published on this topic, providing both short- and long-term outcomes8-11.
The short-term data from the RRISC trial showed that DES (SES) were associated with a reduced in stent late loss, binary in stent and in segment restenosis. Moreover, TLR and TVR were also reduced with DES8. At the long term, DES were associated with a significantly increased risk of death and stent thrombosis whilst the risk of MI and TVR was no longer different between DES and BMS9. Of note, long-term data from SOS trial and Basket trial subgroup did not confirm such a puzzling finding10,11.
The long-term safety of DES is still a matter of debate42-45 and it seems related to the delayed endothelialisation and higher local inflammatory response compared to BMS, but also to an insufficient duration of dual antiplatelet therapy. The latter seems to be critical in particular with respect to the risk of late stent thrombosis. In the registry studies, the duration of dual antiplatelet therapy ranged from three to >6 months (few registries also reported a significant percentage of patients in dual antiplatelet therapy well after 12 months), while in the randomised controlled trials it was two8,9 or six months10,11. Overall, the incidence of stent thrombosis was very low in both BMS and DES arms.
Currently, after DES implantation dual antiplatelet therapy should be continued for at least 12 months if well tolerated6, thus the answer to the question of the long-term safety still requires a properly designed trial.
Limitation of the present study
A limitation inherent to all meta-analyses is the potential heterogeneity among studies, in terms of protocols, patients, and sample sizes, and the unavailability of patient-level data. However, the primary disagreement that arises in meta-analyses is whether to incorporate between-study variation (heterogeneity and inconsistency) in estimating summaries of effect size. In presence of significant heterogeneity it may be more appropriate to analyse results using both methods.
A statistically significant result with the fixed effect model indicates that there is an effect in at least one of the studies, and the overall result is an average measure of treatment effect of the studies in the analysis.
On the other hand, the random effects tends to give a more conservative estimate (i.e., with wider confidence intervals) which has to be preferred in the presence of significant heterogeneity.
The retrospective designs of most of the included studies, the lack of adjusted ORs in some reports, some discrepancies in duration of dual antiplatelet therapy, follow-up, use of protection devices and, ultimately, the use of different DES have to be acknowledged as possible limitations of this analysis. They are all impossible to overcome due to the design of the included studies. Ultimately, the potential risk of selection bias is unavoidable in registry studies.
Avenues for future research
The treatment of SVG disease is a complex scenario where the outcome still remains poor when compared to the treatment of native coronary disease. Despite the initial concerns about DES safety, the present systematic quantitative review does not confirm the issue of an increased mortality after DES. On the other side, the possible advantages of DES seem to be evident at short term follow-up, while they tend to disappear at longer term and in any case they seem to be much less manifest (in terms of absolute and relative reduction) than in native arteries. Properly powered and specifically designed randomised trials, with extensive use of adjunctive devices and long-term dual antiplatelet therapy are required in order to clarify such a complex matter. Moreover, the fact that the RRISC trial (with SES) was very negative while the SOS (with PES) was encouraging may lead to the concept that DES in SVG “are not created equal” therefore suggesting the need for a randomised controlled trial specifically assessing this issue.
Two multicentre studies are currently recruiting patients in Europe. The first one is the Prospective, Randomized Trial of Drug-Eluting Stents vs. Bare Metal Stents for the Reduction of Restenosis in Bypass Grafts (ISAR-CABG) trial (NCT00611910), which plans to enrol 600 patients in two centres in Germany. Patients will be randomised to either a DES arm (three DES will be used, SES, PES or a local polymeric stent coated with rapamycin) or a bare metal stent arm. The primary endpoint is the composite of death, myocardial infarction and target lesion revascularisation at one year after stent implantation. The estimated completion date is April 200946.
The second is the BAsel Stent Kosten Effektivitäts Trial - SAphenous Venous Graft Angioplasty Using Glycoprotein IIb/IIIa Receptor Inhibitors and Drug-Eluting Stents (BASKETSAVAGE) (NCT00595647). BASKETSAVAGE will randomise 240 patients to a paclitaxel-eluting stent (TAXUS® Liberte®; Boston Scientific Corporation, Natick, MA, USA) vs. a similar bare-metal stent (Liberte®; Boston Scientific Corporation). Enrolment will occur at one centre in Switzerland and one centre in Germany. The primary endpoint of the study is the composite of cardiac death, non-fatal myocardial infarction, and TVR and results are anticipated in April 200947.
Conclusion
In our knowledge, there is no previous publication concerning a pooled analysis of all available data investigating the impact of DES compared to BMS in treating SVG disease. Limitations are present, however, pooling results from different observational and randomised studies, with different operators in independent centres can provide a reliable picture of the “real world” scenario. Furthermore, the evidence of some discrepancies with respect to the sensitivity analyses also support the utility of comprehensively pooling the data. Of note, our data are quite consistent with those coming from the comparison of DES vs. BMS in the setting of STEMI patients. This similarity with another high risk group strengthens the reliability of the data.
In conclusion, although an advantage in terms of repeated revascularisations seemed evident at short term, considering the nature of the available studies and the heterogeneity of the data, caution is required before advocating a routinely application of this technology to saphenous vein graft disease.
Importantly, while waiting for higher quality data, the results of our analyses do not confirm previous reports on major issues related to safety of DES in this “off label” indication.
Appendix: algorithm for electronic search on Pubmed*
(randomised controlled trial[pt] OR controlled clinical trial[pt] OR randomised controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR (clinical trial[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind[tw])) OR (latin square[tw]) OR placebos[mh] OR placebo*[tw] OR random*[tw] OR research design[mh:noexp] OR evaluation studies[mh] OR follow-up studies[mh] OR prospective studies[mh] OR cross-over studies[mh] OR control*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (animal[mh] NOT human[mh]) NOT (comment[pt] OR editorial[pt] OR meta-analysis[pt] OR practice-guideline[pt] OR review[pt])) AND saphenous vein graft AND stent.
* Biondi Zoccai GG, Agostoni P, Abbate A, Testa L, Burzotta F. A simple hint to improve Robinson and Dickersin’s highly sensitive PubMed search strategy for controlled clinical trials. Int J Epidemiol 2005;34:224-5.
Acknowledgements
This study is part of a senior investigator project of the Meta-analysis and Evidence-based Medicine Training in Cardiology (Metcardio), based in Oxford, United Kingdom (http://www.metcardio.com).

