Abstract
Aims: PCI with drug eluting stents (DES) has been shown to reduce restenosis and major adverse cardiac event (MACE) rates compared to bare metal stents (BMS) in native coronary vessels, although outcomes in saphenous vein graft (SVG) lesions are less clear. We retrospectively studied 388 consecutive patients admitted to our centre for SVG PCI to assess mortality and MACE outcomes (defined as composite endpoint of all-death, stroke, myocardial infarction, stent thrombosis and target lesion (TLR) / vessel (TVR) revascularisation) associated with BMS and DES use.
Methods and results: Two hundred and nineteen (219) patients had BMS and 169 had DES (total 388 patients). Mean follow up was 41.9±23.5 months. No significant differences were observed in mortality (14.2% vs. 11.8%) or MACE (37.6% vs. 35.8%) between the BMS and DES groups at four years follow-up or at other intervening time points studied. Similarly, no differences in TVR / TLR rates were observed over a similar time period (19.8% vs. 21.6%).
Conclusions: We have observed that DES and BMS use in SVG PCI have comparable mortality and MACE rates, and that in contrast to PCI in native coronary arteries, DES do not reduce revascularisation rates in our study cohort.
Introduction
Both large randomised multicentre trials1,2 and registry studies3,4 have demonstrated that percutaneous coronary intervention (PCI) with drug eluting stents (DES) is associated with reduced restenosis and major adverse cardiac event (MACE) rates compared to bare metal stents (BMS) in native coronary arteries. Despite this body of evidence for lesions in native coronary arteries, lesions in saphenous vein grafts (SVG) have either been excluded or poorly represented in pivotal DES trials. SVGs degenerate over time with 40% occlusion rates within 10 years5 and of those grafts that do not occlude, 43% will have significant stenosis (>50%). PCI has surpassed CABG as the treatment of choice for SVG disease6. However, the use of bare metal stents (BMS) in SVG is associated with target lesion revascularisation rates as high as 20% at one year7 and this has prompted a move towards the use of DES in SVG PCI. Recent data evaluating DES in native coronary arteries have raised concerns regarding late stent thrombosis and myocardial infarction2,8,9. In addition, delays in endothelial healing after DES implantation in SVGs in addition to enhanced local pro-thrombotic conditions10,11 have raised concerns regarding potential increased risks of acute, sub-acute and late stent thrombosis using DES in this setting. The recent DELAYED RRISC trial demonstrated that BMS were associated with lower long-term mortality than DES in SVG procedures with no differences in the rates of myocardial infarction and repeat vascularisation procedures12. In contrast, in the SOS trial, mortality rates were similar, although target vessel revascularisation and binary angiographic restenosis rates were decreased in the DES group in comparison to the BMS group13. Current registries also report inconsistent results regarding the outcomes of DES use in SVG PCI and are limited by small enrolment numbers and follow-up data7,14-20. The issue regarding outcomes of DES vs. BMS in SVG may become more pertinent following publication of the VELETI pilot trial that demonstrated that stenting moderate non-significant lesions in SVGs with DES was associated with a lower rate of SVG disease progression and a trend toward a lower incidence of MACE at 1-year follow-up compared to medical treatment21. Consequently, the aim of this study is to assess outcomes in a consecutive series of a large cohort of patients treated with DES and BMS for lesions in SVG.
Methods
We retrospectively studied 388 consecutive patients admitted to Manchester Heart Centre for PCI to SVG lesions from 2001 to 2008. BMS were used throughout the study period, although DES use began at our centre in April 2002. All elective patients were referred for PCI based on clinical symptoms or inducible ischaemia documented by non-invasive stress testing.
PCI was performed according to the practice of the individual operator at our institution in line with best evidence-based practice available at that particular time. All patients were pretreated with aspirin and clopidogrel. PCI was performed with heparin anticoagulation by all operators, but the PCI strategy employed, including balloon pre-dilation of the target lesion, the use of mechanical devices, selection of BMS or DES and the adjuvant use of GPIIb/IIIa inhibitors were all left to the operator’s discretion. Patients receiving a BMS were treated with clopidogrel for at least one month whereas those receiving a DES were treated for at least 6-12 months. All patients were advised to remain on aspirin indefinitely.
Clinical endpoints studied included all-cause mortality and major adverse cardiac events (MACE) defined as the composite endpoint of all cause mortality, stroke, myocardial infarction (MI), stent thrombosis and target lesion/vessel revascularisation. Mortality data were obtained from the National Office of Statistics. Repeat revascularisation procedures, episodes of MI and complications were collected prospectively from the Manchester Heart Centre PCI database in which procedural and clinical and demographic data are entered for each patient undergoing PCI, prospectively and retrospectively. Data quality entered into the database was cross-checked and validated by an independent Clinical Information Assistant using the PCI procedural reports generated by the operator and information obtained from the medical notes. For patients admitted to peripheral hospitals in the acute phase, the diagnosis of MI was confirmed by documentation from the referring physician. MI was defined as any typical rise above the upper range limit and fall of biochemical markers of myocardial necrosis with at least one of the following: cardiac symptoms, development of Q waves on the ECG, or ECG changes indicative of ischaemia.
TVR/TLR was defined as a clinically driven or ischaemia testing driven revascularisation of the index graft / lesion. Stent thrombosis was defined as angiographically confirmed thrombosis with partial or total thrombotic occlusion of the peri-stent region accompanied with an acute clinical presentation (acute ischaemic symptoms or ischaemic ECG changes or elevated cardiac biomarkers) as per ARC definition of definite stent thrombosis22.
Patient demographics were obtained from the Manchester Heart Centre patient database. Diabetes was defined as per WHO criteria or treatment with either oral hypoglycaemic agents or insulin.
Statistical analysis
Continuous variables are presented as mean±standard deviation. Fischer’s exact tests were used for analysis of categorical variables and Student’s t-tests were used to analyse continuous variables. The relationship of baseline variables with mortality and MACE was assessed with Cox proportional hazards regression with multivariable analysis. Factors thought to be important for the endpoints were entered for the analysis and this model included: age, sex, diabetes, glycoprotein IIb/IIIa inhibitor use, distal protection device use, stent size, lesion length, ejection fraction, presence of thrombus, clinical presentation and type of stent used. Kaplan-Meier cumulative survival and MACE curves were constructed and compared by the log-rank test. All statistical tests were two-tailed. A value of p<0.05 was used to indicate statistical significance. Statistical analysis was performed using MedCalc version 10.4 (MedCalc Software, Mariakerke, Belgium) and Kaplan Meier curves were constructed with Prism 5 for Mac (GraftPad Software Inc., La Jolla, CA, USA).
Results
A total of 388 patients underwent PCI to SVG lesions during the study period; with 219 patients receiving BMS and 169 receiving DES. Mean follow-up of the patient cohort was 41.9±23.5 months, although patients in the BMS group had a significantly longer follow-up compared to those in the DES group (49.5±23.9 months versus 32.0±18.8 months, respectively; p<0.0001). A comparison of the clinical demographic data of those who underwent SVG PCI with BMS versus DES use is presented in Table 1.
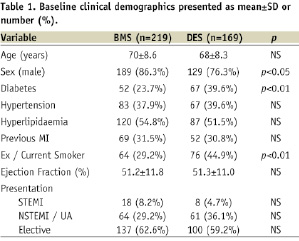
The mean age of the two groups was similar, but there was a higher proportion of males (86.3% vs. 76.3%) in the BMS group and a higher prevalence of diabetes (39.6% vs. 23.7%) and history of smoking (44.9% vs. 29.2%) in the DES group. Of those patients treated with DES, 107 patients (63.3%) were treated with Taxus stents (Boston Scientific, Natick, MA, USA), 28 patients (16.6%) were treated with Cypher stents (Cordis, Johnson & Johnson, Warren, NJ, USA), 18 (10.6%) were treated with Endeavor stents (Medtronic, Minneapolis, MN, USA) and 11 (6.5%) treated with Promus stents (Boston Scientific).
Lesion characteristics and procedural data are presented in Table 2.
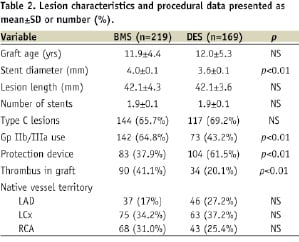
The mean graft age was 11.9±4.4 years in the BMS group and 12.0±5.3 years in the DES group. A similar number of stents were utilised in both groups (mean 1.9 stents) and mean stent length was 42.1 mm in both groups, although the BMS group had a larger mean stent diameter than the DES group (4.0 mm vs. 3.6 mm, p<0.01). Furthermore there was greater use of GP IIb/IIIa inhibitors in the BMS group (64.8% vs. 43.2%; p<0.01), but more embolic protection device use in the DES group (61.5% vs. 37.9%; p<0.01).
Cumulative mortality rates from 30 days to four years are presented in Table 3.
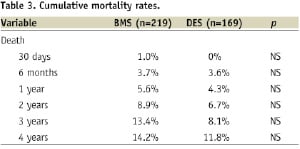
Figure 1 illustrates Kaplan-Meier survival curves for both the BMS and DES groups, with no statistical difference in survival curves observed between the two groups; (p=0.33 Log-rank test; HR 1.38, 95% CI 0.74-2.60).
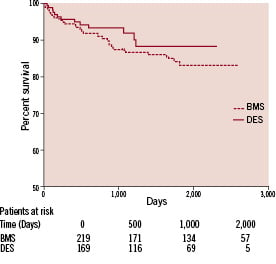
Figure 1. Kaplan-Meier Curve for mortality.
Furthermore, no statistical difference in mortality between BMS and DES was observed for SVG vessel diameter <3.5 mm (p=0.09; HR 3.2, 95% CI 0.84-12.22) or >3.5 mm (p=0.90; HR 0.95, 95% CI 0.44-2.04). Cox proportional hazards regression analysis adjusted for age, diabetes, sex, clinical presentation, LV function, distal protection device, lesion length, stent size, presence of thrombus and Gp IIb/IIIa use was performed, with multivariate analysis revealing no mortality benefit associated with DES use (HR 0.93, 95% CI 0.68-1.88).
Cumulative MACE rates and TLR/TVR rates from 30 days to four years, presented in Tables 4 and 5, respectively, were similar between the two groups.
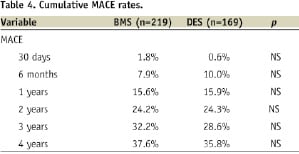
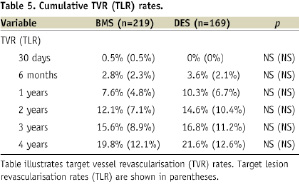
Figure 2 illustrates Kaplan Meier curves for freedom from MACE events and demonstrates that the event rates were similar in both groups (p=0.82 Log-rank test; HR 0.96, 95% CI 0.67-1.37).
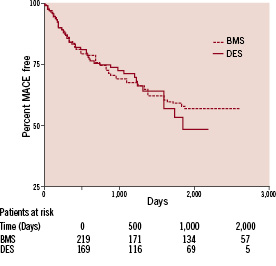
Figure 2. Kaplan-Meier Curve for MACE.
Furthermore, no statistical difference in MACE was observed between BMS and DES for SVG vessel diameter <3.5 mm (p=0.053; HR 2.07, 95% CI 0.99-4.32) or >3.5 mm (p=0.07; HR 1.13, 95% CI 0.72-1.93). Cox proportional hazards regression analysis adjusted for age, diabetes, sex, clinical presentation, LV function, distal protection device, lesion length, stent size, presence of thrombus and Gp IIb/IIIa use was performed, with no MACE benefit associated with the use of DES; HR 1.22 (95% CI 0.43-2.01).
Discussion
In one of the largest series published to date with over four years of follow-up data, we have observed that the use of DES in SVG PCI is safe and not associated with excess mortality or MACE rates compared to BMS use. Furthermore, although DES use is widely known to reduce TLR and TVR rates when treating native coronary arteries, our study showed no such therapeutic advantage of DES over BMS use when treating SVG disease.
Previous reports have yielded inconsistent mortality and MACE outcomes comparing the use of DES and BMS in SVG PCI. Data from the DELAYED RRISC Trial consisting of 75 patients with a median follow-up of 32 months demonstrated an increased risk of mortality associated with DES12 with a 29% six month mortality rate in the DES arm versus 0% in the BMS arm. Indeed, numbers needed to harm analysis illustrated treatment of 3.4 patients (95% CI 2.2 to 7.3) with SES resulted in one additional death with respect to treatment with BMS. Angiographic follow-up at six months revealed less in-segment restenosis in the DES arm with an associate reduction in six months TVR rates (5.3% versus 27%), but this benefit was lost at 32 months median follow-up.
In contrast, in a randomised multicentre study involving clinical and angiographic follow up of 120 patients over six months, MACE rates at six months were significantly lower in the DES cohort, driven mainly through a reduced TLR rate23, although in this study the Cook paclitaxel-eluting stent was used which has higher late loss than other DES and is no longer commercially available. Similarly in the SOS trial, TVR and binary angiographic restenosis rates were decreased in the DES group in comparison to the BMS group, although mortality rates were similar13.
It is unclear why DES mortality rates in the DELAYED RRISC study were so much greater than in the BMS arm. This unexpected result significantly differs from previously published 6-month SVG PCI mortality rates of 0-7% and 2.2%-8% mortality rates7,16,20,24 reported in DES and BMS arms, respectively. Similarly our mortality rates at six months were 3.7% and 3.6% in the BMS and DES cohorts, respectively. Of the 11 deaths in the DES arm in the DELAYED RRISC study, seven were cardiac deaths of which three were sudden possibly related to late or very late stent thrombosis and one additional death was due to an angiographically documented very late stent thrombosis resulting in a large AMI and death. Interestingly, clopidogrel use in the DES arm of this trail was only necessitated for two months, way may in part explain this excess mortality.
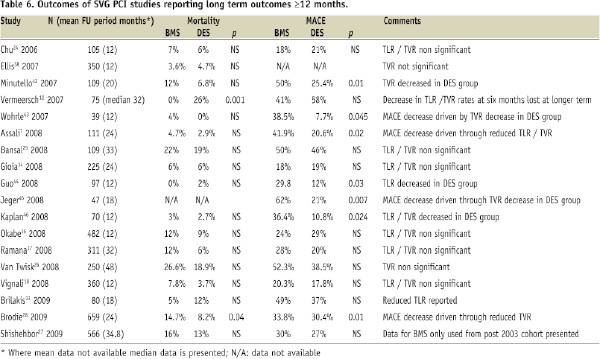
In registry studies, data have been inconsistent with studies showing either no difference7,14,16,24-27 or reduced mortality rates15,28 between DES and BMS. Similarly both a reduction7,15,17,20 and no difference14,16,18,24-27,29 in MACE rates have been observed. Furthermore, in the study of Shishehbor et al27 no differences in mortality or MACE outcomes were observed irrespective of whether BMS data was used pre or post 2003. In studies where a decreased MACE rate in the DES cohort was observed7,13,15,17,20,23 this was mainly driven by a reduction in TVR7,13,15,17,20, TLR23,7,17,20 or myocardial infarction15. Table 6 summarises outcomes of SVG PCI studies reporting long term outcomes ≥12 months.
It has been previously argued that the lack of benefit observed in MACE or TLR/TVR rates associated with the use of DES may be related to the fact that some of these studies were small with the inclusion of 100-150 patients7,24,25, or that the studies were of short follow-up periods of up to 12 months16,18,24. However, our more extensive study reinforces the observed lack of benefit associated with DES use in MACE or TVR/TLR rates.
It is unclear why the use of DES in SVG is not associated with an improvement in TLR/TVR rates since the mechanism of in-stent restenosis in vein grafts is similar to that observed in native coronary arteries through neointimal hyperplasia30. In contrast, histological studies10 suggest important differences in the response of SVG to PCI compared to native vessels, for example there is often an aggressive neointimal response following PCI with the persistence of inflammatory cells around the areas of stent deployment for periods of time up to five years following PCI. It also appears that organised thrombus represents the main component of the lumen narrowing material within SVG, with the larger diameter and the lower elasticity of the vein conduit resulting in slower flow thereby promoting platelet apposition and thrombus formation hence subsequent restenosis. Consequently DES would not be expected to reduce rates of re-stenosis occurring through these mechanisms.
Vessel size is also an important determinant of restenosis with low rates of restenosis (<10%) after BMS implantation in large coronary arteries31,32. Registry data suggest that in large native coronary arteries (>3.5 mm), no significant differences in 12-month mortality, MACE or TVR/TLR rates are observed between DES and BMS use33. Data from the National Heart, Lung, and Blood Institute Dynamic Registry comparing BMS and DES use for off-label indications in 6,551 patients, DES use in vessels ≥3.75 mm in diameter was not associated with reduced revascularisation rates34. Similarly, trials such as TAXUS-IV and V, suggest that the benefit of DES is limited to vessels ≤3 mm35,36. In the randomised BASKET trial, DES conferred no benefit in large native vessels ≥3 mm in reducing TVR rates (HR 0.75, p=0.38)37. Similarly, given that the mean SVG diameter was 3.6 mm in the DES group and 4 mm in the BMS group in our study, one might not expect to see an additional benefit from DES use since TLR /TVR rates observed in the BMS group were relatively low (7.6% at one year). In support of such a view, in the multicentre U.S. STENT registry evaluating outcomes with DES, analysis of SVG PCI revealed that DES reduced TVR at nine months in SVG lesions with diameter <3.5 mm (8.0% vs. 17.2%, p=0.013) but not ≥3.5 mm (6.0% vs. 6.6%, p=0.74)28. In contrast, the study of Shishehbor et al27 did not reveal any differences in the composite outcome death, MI, or TLR between patients with vessel diameters either <3.5 mm (HR 0.36 (0.12-1.05) or ≥3.5 mm (HR 1.28 (0.26-6.46). Similarly we have not seen any differences in either mortality or MACE rates between DES and BMS in SVG diameter either < or ≥3.5 mm.
Our 1-year TLR/ TVR rates of 7.6% in the BMS group are lower than some comparative studies, for example Chu et al24 reported one year TLR rates of 11%, whilst Ellis et al38 reported rates of 11.8%, hence the lower BMS event rate in our study may explain why no significant differences were observed in revascularisation rates between BMS and DES in our study. However, our 1-year revascularisation rates are in line to those of Okabe et al16 8% and Vignali et al18 8.1% who similarly did not report differences in revascularisation rates between BMS and DES in their studies. Another possible reason for the lack of benefit of DES vs. BMS observed in SVG lesions may relate to the rapid progression of atheromatous disease within SVG. For example, in the recent VELETI pilot study, 22% of moderate SVG lesions (defined as 30-60% diameter stenosis) progressed to severe flow limiting lesions or vessel occlusion within one year, hence a benefit of DES on TVR my not be observed due to the rapid progression of other non significant lesions21.
There are a number of potential limitations to this study, including its inherent retrospective nature which has all the limitations associated with studies of this kind. Furthermore, this is a “real world” observational study in which the selection of patients, the decision to intervene and choice of technique and equipment used were at the operators’ discretion and so may not reflect cohorts used in randomised controlled studies. In addition, patients did not systematically undergo angiography and so differences in restenosis rates per se between the two groups could not be accurately assessed and silent occlusions of the vein grafts could not be detected. Furthermore DES use was not introduced at our centre until 2002, hence patients undergoing PCI with BMS had significantly longer follow-up. Finally, current guidelines recommend that dual antiplatelet therapy should be continued for at least 12 months in patients receiving DES39. Although the majority of our patients treated with DES would have received dual antiplatelet therapy for at least 12 months, it is likely that those receiving DES before 2004 would have received dual antiplatelet therapy for significantly shorter periods of time reflecting contemporary practice at that time40,41. Despite these potential limitations, the large numbers and long duration of follow-up associated with our study would suggest that our findings are relevant to real world practice.
Summary
In one of the largest registry studies to date with one of the longest follow-up periods, we have demonstrated similar mortality and MACE rates in patients undergoing SVG PCI using either DES or BMS. Furthermore, in contrast to the observed reduction in TLR and TVR rates in native coronary arteries, we have observed no differences between TLR and TVR rates between BMS and DES in SVG PCI. Given that SVG PCI cases represent 5-10% of all PCI cases performed, and that SVG disease has been excluded from pivotal DES versus BMS trials, further adequately powered large randomised trials are needed to clarify the role of BMS and DES in SVG interventions.

