We have read with interest the article written by Brilakis et al1 related to the use of drug-eluting stents in vein grafts. As the authors mentioned, the information available so far is heterogeneous. We agree, with them, that this information can most likely be summarised by the following: that both bare metal and drug-eluting stents provide similar survival rates, that there are significant differences in late loss, and that the differences in revascularisation are influenced by the angiographic follow-up. However, we believe that there is one fact that should be examined in detail if we want to advance in this field: benefits of drug-eluting stents in native vessels are especially demonstrable in diameters ≤3 mm2,3, in vein grafts this variable may have singular relevance. In a published series of 236 lesions in vein grafts in Spain, stents ≥3.5 mm represented 62.7% of the total, a percentage much higher than in studies of native vessels4. The fact that we need to refer to the angiographic follow-up to demonstrate the reduction in target vessel revascularisation in saphenous grafts, and the note of caution concerning the increase in mortality with drug-eluting stents in the DELAYED RRISC5 trial, requires we carefully select lesions that might benefit from this procedure. In our opinion, the design of the studies and the analysis of results in saphenous vein grafts should take into account the vessel size. We believe that it would be probably desirable to analyse – separately – two groups with diameters above and below 3.5 mm. We believe that the future results of the forthcoming DIVA trial with its prospective, blinded multicentre design with clinical endpoints, along with the analysis in relation to stent diameter mentioned in this letter, will contribute to advancing this still confusing field.
We greatly appreciated Dr. Lozano’s insightful comments on our review on the outcomes after drug-eluting stent (DES) implantation in saphenous vein grafts (SVGs).1 We agree that the SVG reference diameter may play an important role in the risk for subsequent adverse outcomes, yet this information is infrequently reported in published studies. As Dr. Lozano highlighted, there are limited published data on whether bare metal stents (BMS) suffice for large diameter SVGs (whether defined as >3.0 mm or >3.5 mm).
Brodie et al recently reported the DES reduced target vessel revascularisation at nine months in SVGs with diameter <3.5 mm (8.0% vs. 17.2%, p = 0.013) but not in SVGs with diameter 3.5 mm (6.0% vs. 6.6%, p = 0.74) among 785 DES and 343 BMS patients participating in the STENT (Strategic Transcatheter Evaluation of New Therapies) registry.2 The SVG reference vessel diameter was <3.5 mm in 41.7% of the DES and 25.8% of the BMS group.
Registries have significant limitations when trying to assess the efficacy of novel devices. To overcome these limitations we examined the SOS (Stenting Of Saphenous vein grafts) trial database3 to determine whether BMS implantation was associated with good outcomes after stenting of large SVGs. Among 112 SVG lesions included in SOS, 47% and 66% had reference vessel diameter ≤3.0 mm or <3.5 mm, respectively. Binary in-segment angiographic restenosis at 12 month follow-up angiography (available for 90 lesions) was significantly higher among DES-treated lesions in both ≤3.0 mm (65% vs. 21%, p=0.005) and >3.0 mm SVGs (41% vs. 0%, p<0.001) (Figure 1).
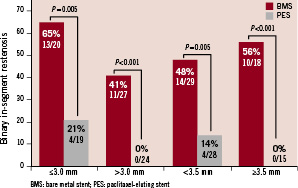
Figure 1. Binary in-segment angiographic restenosis among 90 lesions with 12-month angiographic follow-up from the SOS (Stenting Of Saphenous vein grafts) trial, classified according to the saphenous vein graft reference diameter.
Similar results were seen in <3.5 mm (48% vs. 14%, p=0.005) and ≥3.5 mm (56% vs. 0 %, p<0.001) SVGs (Figure 1). During a median follow-up of 18 months the incidence of target lesion revascularisation was lower with DES in ≤3.0 mm (31% vs. 0%, p=0.005) and >3.0 mm (36% vs. 13%, p=0.25) SVGs. Similarly, the incidence of target lesion revascularisation was lower with DES both in SVGs with diameter <3.5 mm (40% vs. 9%, p=0.05) and those with diameter ≥3.5 mm (28% vs. 0 %, p=0.03) (Figure 2).
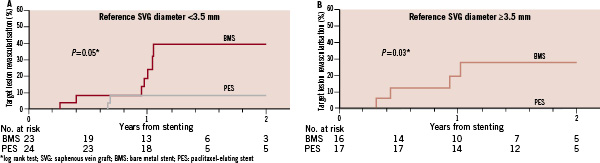
Figure 2. Incidence of target lesion revascularisation among patients who received a paclitaxel-eluting stent (PES) vs. a similar bare metal stent (BMS) in the SOS trial, classified according to the target saphenous vein graft reference diameter.
Therefore, based on the SOS trial findings, BMS implantation in large SVGs (whether 3.0 mm or 3.5 mm is used as the cutoff) appears to carry significant risk for developing in-stent restenosis and to require repeat target lesion revascularisation, whereas paclitaxel-eluting stents significantly reduce that risk. Considering the grave consequences of stent failure in SVGs –frequent presentation with an acute coronary syndrome and with SVG occlusion4 – and until data from large randomised-controlled trials become available,1 we would argue against using SVG size as a criterion for favouring BMS use in SVGs.

