Abstract
Saphenous vein grafts (SVG) frequently develop atherosclerotic disease that may result in stenosis or occlusion. Percutaneous coronary intervention (PCI) of SVG is associated with a relatively high-risk of procedural complications (due mainly to distal embolisation) and restenosis rates, that along with accelerated atherosclerotic disease progression, result in a poor mid-term outcome. The role of drug-eluting stents (DES) in improving clinical results of PCI in SVG has not yet been established.
Current information is limited and based on several retrospective uncontrolled cohort studies comparing DES and bare metal stents (BMS), two matched case-control studies and two prospective, very small size single-centre trials (RRISC, SOS) designed for 6-month angiographic endpoints, but underpowered for clinical events.
Most studies with a short follow-up reported a significant reduction in target vessel revascularisation (TVR) in the DES-treated group, but for those with longer follow-up periods, the differences were either smaller or non-significant. Regarding safety, only the DELAYED RRISC trial reported a significant increased in stent thrombosis and death rates, while no increase in mortality was observed in any other. In fact, in two studies a trend towards a lower mortality rate was detected.
In light of the actual data, and until more evidence has been provided by properly sized, multicentre prospective, randomised trials (which not even ongoing yet), we may assume that DES decrease short-term TVR rates as they do in native vessels, but that their impact on clinical events after one year is weak and inconsistent. Although unproven, the increase in mortality reported in one single trial supports a cautious approach towards the use of DES in SVG. Whether specific stent platforms, polymers or drugs are more appropriate in SVG lesions has not been addressed at this time.
Abbreviations
ACR: Academic Research Consortium
BMS: bare metal stent
DES: drug-eluting stent
CABG: coronary artery by pass graft
IVUS: intravascular ultrasound
LAD: left anterior descending coronary artery
LIMA: left internal mammary artery
MACE: major adverse cardiac events
MI: myocardial infarction
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent
R: restenosis
SVG: saphenous vein graft
SES: sirolimus-eluting stent
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Background
Saphenous vein grafts (SVG) have been extensively used for some time now in coronary artery bypass surgery (CABG) as additional conduits to arterial grafts. However, within a few years, they develop atherosclerotic disease that may result in stenosis or occlusion of the graft1. Around half of the millions of patients treated with these conduits over the last 10 years will be presenting in the next few years with graft attrition, and many of them will require secondary revascularisation. Reoperation of these patients, who usually have patent arterial conduits, is considered feasible, but is associated with higher mortality rates and poorer clinical outcome than if it was their first operation.
Balloon angioplasty in SVG stenosis was an alternative to redo-CABG, but a combination of procedural complications (due mainly to distal embolisation), a high restenosis rate and associated atherosclerotic disease progression in the graft were responsible for a poor mid-term outcome of percutaneous coronary interventions (PCI).
Several procedural improvements, such as the use of stents2,3, distal embolisation protection devices, IIb/IIIa inhibitors and direct stenting technique, have contributed to better initial angiographic and clinical outcomes, but midterm results4,5 are still not comparable to those of native vessels. Data on the role of drug-eluting stents (DES) and their possible contribution to reduced clinical events in native vessels is still lacking.
The purpose of this review is to analyse the available data addressing the use of DES in PCI of SVG.
Post-CABG vein graft failure
Postoperatively, about 10% of grafts are occluded early, 20% by the end of the 1st year and up to 50% within five to 10 years after CABG. In addition, by the 10th year, 70% of patent grafts are diffusely diseased1. Three processes are involved in graft failure: 1) thrombosis is responsible for very early graft occlusion and, assuming optimal surgical technique, may be prevented by antithrombotic/antiplatelet drugs, 2) intimal hyperplasia causes most failures occurring between 30 days and one year. This problem is due to endothelial damage induced during vein harvesting, loss of intrinsic vascular supply and the abrupt increase in wall stress with under high pressure conditions. 3) the development of atherosclerotic plaques is the main mechanism of “old” graft failure. Plaques are usually concentric, diffuse, lipid-rich and with a high content in thrombotic material and inflammatory cells and absence of fibrous cap. All these features make SVG lesions to be very friable and prone to thrombotic occlusion and spontaneous or PCI-induced distal embolisation.
PCI of venous conduits
According to large registries, PCI of SVG accounts for 2 to 10% of all PCI procedures.
Even with the use of distal protection devices, direct stenting technique and potent antiplatelet agents, PCI of vein grafts is associated with the relatively high risk (10-20%) of non-reflow phenomena and its clinical consequences (myocardial necrosis and increased death rate) compared to native vessels interventions. In addition, the rate of restenosis (R) is not only increased, but also delayed (>12 months) as compared with R in native coronary arteries. Histopathological changes of restenotic SVG segments6 involves not only proliferation of smooth muscle cells and collagenous matrix, as in native vessels, but also accumulation of foam cells, extracellular lipids, cholesterol crystals and inflammatory cells, typical features of human atherosclerotic plaques. Furthermore, disease progression in non-treated segments is frequent in old grafts and may accounts for about one half of late major adverse clinical events (MACE) and represents a confounding factor in the assessment of PCI long-term result.
Assessment of PCI results in SVG
For assessment of long term PCI results in native vessels, early (six to nine months) endpoints as angina recurrence, the need for repeat revascularisation or total MACE are appropriate for clinical evaluation while the R rate or late lumen loss by quantitative angiography and intimal hyperplasia by intravascular ultrasound (IVUS) are surrogate non-clinical endpoints that provide relevant information with small sample sizes.
In the case of SVG assessment, longer follow-up periods are required, since R may develop even after one year. In addition, other confounding factors such as atherosclerotic disease progression, either in the lesion, the graft or anywhere in the coronary tree, makes clinical evaluation difficult. Therefore, the R rate, TLR, TVR, clinically driven TLR, clinically driven TVR or total MACE at short-term or long-term, have all been used in the assessment of PCI results in SVG.
DES in SVG
In comparison with BMS, DES have been shown to decrease intimal hyperplasia and R rates in native vessels. In SVG DES also reduce intimal hyperplasia early after stent implantation as evidenced by short-term IVUS studies, but their impact on the development of “new” in-segment atherosclerotic plaques, disease progression in non-treated segments as well as thrombotic occlusion of the graft, have not yet been well defined.
Superiority of DES over bare metal stents (BMS) would require, not only a reduction in non-clinical endpoints (restenosis, neointimal volume), but also a decrease in total MACE (composite of TVR+MI+death) without increase in “hard” endpoints (death, MI) at long-term follow-up (at least 2 years).
Several studies have addressed this issue:
1) Registries of DES in SVG. Several small single-centre series of DES in SVG (Table 1) using SES, PES or both with short-term follow-up have been reported. Restenosis, TVR and total MACE rates were considered relatively low compared with historical series of SVG treated with BMS.
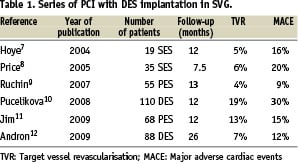
2) Retrospective cohort comparison of DES vs BMS. At least 14 studies13-26 have been reported (Table 2), nine of them favoured DES in terms of TVR rates. From the six studies with more than 100 DES patients16-17,19,24-26, only three16-17,19 with a follow-up period of 9, 12 and 14 months, respectively, found significant differences in favour of DES, while two25,26 (with follow-up of 38 and 48 months) had a tendency towards a reduction in TVR and one24 (with a follow-up of 30 months) did not find any difference. The initial advantage of DES tend to decrease with time in most, but not all26, studies (Figure 1). Not one of the above mentioned studies reported an increase in mortality or MI rates in DES treated patients. In fact in two studies19,25 with 128 and 141 DES patients, a trend toward a reduction in mortality rate was found.
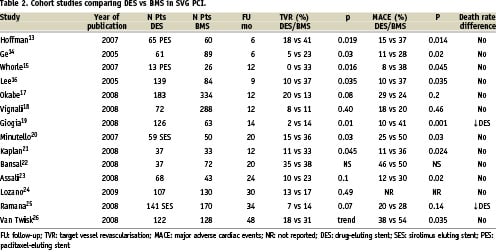
3) Case-control studies. Ellis et al27 in a multicentre study, compared the results of 175 consecutive patients prospectively treated with SES in SVG stenosis and another group of 175 patients obtained from a large database treated with BMS and matched by graft diameter, number of stents deployed and diabetic status. After one year, a modest, non-significant reduction was found in R rates (7.4 vs 13.6%, p=0.08), TLR (6.8 vs 9.9%) and TVR (6.8 vs 11.8%) without differences in death rate (4.7 vs 3.6%).
Applegate et al28 reported on the comparison of 74 consecutive patients treated with DES in SVG and 74 consecutive propensity score matched patients treated with BMS. Covariates included in the propensity score model were age, male gender, heart failure class III-IV, smoking status, diabetes, hypertension, hypercholesterolaemia, vascular disease, renal dysfunction, previous myocardial infarction, number of stents implanted, lesion length, distal embolic protection device, and PCI indicated for a previous restenotic lesion. After two years, TVR was less frequent but without statistical significance in DES group (HR 0.54, 0.21-1.36, log-rank p=0.181). No differences were found in mortality (HR 1.19, 0.32-4,45) or ACR definite or possible stent thrombosis (HR 0.49, 0.09-2.66)
4) Randomised studies.
a) Post-hoc subgroup analysis of the BASKET Trial29. In this single centre, prospective randomised trial of DES vs BMS, the subgroup of patients with native vessels < 3 mm or SVG (47 patients in total) treated with DES was found to have a better clinical outcome than those treated with BMS. Neither the number of SVG patients, nor its outcome, were reported separately from the small vessel subgroup.
b) RRISC Trial30: This prospective, double blind, randomised trial included 75 patients (96 SVG lesions) treated with SES (38 patients) or BMS (37 patients). Clinical, angiographic and IVUS at six months follow-up were obtained. The primary endpoint was late lumen loss at six months angiography; secondary endpoints were clinical events (death, myocardial infarction, TVR), binary R rates (angiography) and intimal hyperplasia volumetric parameters (IVUS). Both groups were well balanced for clinical and angiographic baseline characteristics. Clopidogrel was administered for two months in both groups. At six months, neointimal volume decreased from a median of 24 (0-34) to 1 (0-13) mm3, p=<0.001) as did late lumen loss (mean from 0.70≤0.11 to 0.41≤0.10 mm, p=0.01), R rates (from 33% to 14%, p= 0.031) and TLR (from 20% to 5%, p=0.047). A post-hoc, long-term follow-up was performed after concern about DES safety had arisen. The DELAYED RRISC trial31 (Death and Events at Long-term follow-up AnalYsis: extended duration of Reduction of Restenosis In Saphenous vein grafts with Cipher stent) reported on clinical events up to three years (median 32 months) after the index procedure. An increase in total death rate (Figure 2) in DES patients (29% vs 0% p<0.001) and a catch up of the initial advantage on TVR (34% with DES, 38% with BMS, p=0.38) were observed (Figure 1). There were 11 deaths in DES group and none in the BMS group. Death was non-cardiac in four patients and cardiac in seven, including three sudden deaths, three due to congestive heart failure and one after redo-CABG. Stent thrombosis according to ARC criteria occurred to five patients in DES group (two documented at 14 and 30 months of follow-up, and three possible at 7, 11, 35 months) and none in the BMS group (p= 0.054 [Fisher] and 0.022 log-rank). Considering the results of this trial, it is noteworthy to note that the mortality rate in the BMS group was unexpectedly low compared with the historical BMS series, and that mortality in the DES group was unexpectedly high. As stated by its authors, the small sample size, the short-term dual anti-platelet therapy (two months by protocol) and the post hoc character of the analysis made their conclusions open to confirmation by a properly sized prospective randomised trial.
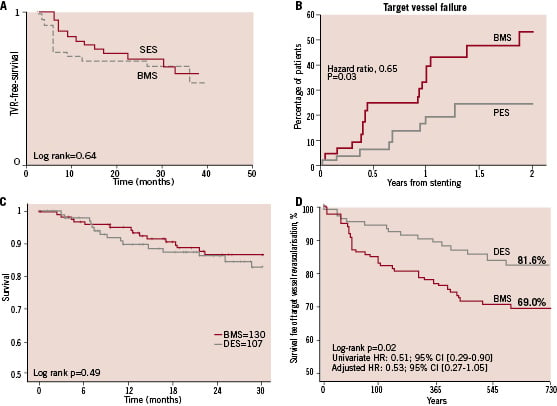
Figure 1. Target-vessel revascularisation temporal trends in several studies. A: DELAYD RRISC trial31. Initial TVR advantage in DES-treated patients is lost later on. B: SOS Trial32. The decrease in TVR is maintained over time. C: Spanish multicentre study24. No impact of DES on TVR from the beginning. D: Thoraxcenter study26. Initial reduction in TVR maintained. SES: sirolimus eluting stent; PES: paclitaxel eluting stent; BMS: bare metal stent; DES: drug eluting stent; TVR: target vessel revascularisation.
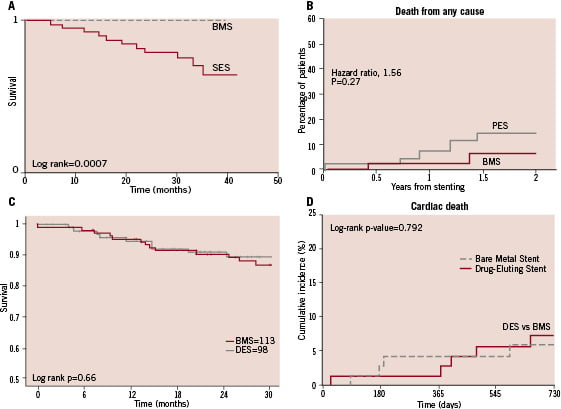
Figure 2. Temporal trends in death rates after DES and BMS implantation in SVG in several studies. A: DELAYD RRISC trial31. B: SOS Trial32. C: Spanish multicentre study24. D: Applegate et al study28. Significant increase in death rate was only reported by DELAYED RRISC trial (panel A). SES: sirolimus eluting stent; PES: paclitaxel eluting stent; BMS: bare metal stent; DES: drug eluting stent; TVR: target vessel revascularisation.
c) The SOS (Stenting of sapheous vein grafts) trial32 is a multicentre, prospective, randomised trial that included 39 patients treated with PES in SVG and 45 patients treated with BMS. Clinical and angiographic baseline and procedural variables were well balanced. After 24 months, TVR was lower in the DES group (15 vs 44%, p=0.005) without differences in total MACE (37 vs 49% p=0.20), death o MI rates.
DES in lesion subtypes
Long lesions. Diffuse atheromatous involvement is very common in saphenous vein grafts. Andron et al12 analysed PCI results in 88 patients with lesions >50 mm in SVG (mean length 70 mm [51-135] treated with 2.7 [2-5] DES per graft). After a mean of 26.5 (6-60) months, one patient died, eight had a MI, two stent thrombosis (at 14 and 17 months) and six required TVR. They concluded that in this lesion subset DES use is reasonably safe and effective.
Ostial lesions. Kaplan et al21 reported on 70 patients with ostial SVG lesions, 37 of whom were treated with DES and 33 with BMS. After one year, the BMS group had a higher TLR (30% vs 5%, P=0.015), TVR (33% vs 11%, P=0.045) and MACE rate (36% vs 11%, P=0.024) compared to DES group. Their conclusion is that in ostial SVG lesions DES provides better clinical results than BMS.
Mild to moderate lesions. The VELETI trial (sealing moderate coronary saphenous VEin LEsions with the paclitaxel-eluting stent Taxus as a new approach to maintain vein graft patency and reduce cardiac events: a pilot Intravascular ultrasound study) is a randomised trial comparing DES with medical treatment in patients with moderate SVG stenosis. The study is already completed (80 patients) but results have not been released yet.
SES vs PES
Very scarce information is available concerning potential differences in DES implanted SVG. Chu et al33 from Washington Medical Center reported on 47 patients with 50 SVG treated with SES and 42 patients with 45 SVG treated with PES. There were no differences in acute results, immediate complications or 6-month MACE rate (p=0.75). There were no cases of stent thrombosis in any group.
Should we treat SVG with DES?
The current information available on the comparison of DES and BMS implantation in SVG is limited and based on several retrospective uncontrolled cohort studies, two matched case-control studies, and two prospective, very small size (38 and 39 patients in DES group respectively) single-centre trials designed for 6-month angiographic endpoints (late lumen loss in the case of RRISC and binary R rate in the SOS trial). These trials were too underpowered to draw conclusions about clinical events.
Most studies with a follow-up of less then one year reported a significant reduction in TVR in DES-treated group13-17,21, but others with longer periods of follow-up periods found such a difference to be either smaller or non-significant22-24,26. Among studies reporting temporal trends in TVR, differences between groups decreased or disappeared after two years28,31, but in two studies, the initial advantage is maintained over time26,32 (Figure 1).
Regarding concerns about long-term safety of DES35-37, only the DELAYED RRISC trial reported a significant increased in stent thrombosis and death rate in the DES group, with an unexpectedly low mortality rate in BMS group. No increase in mortality was observed in any other study and in fact two of them even evidenced a trend toward a decreased mortality rate19,25.
In light of the actual data, and until more evidence is provided by properly sized, multicentre, prospective, randomised trials (which are not even ongoing yet), we may assume that DES decreases short-term R and TVR rates as it does in native vessels, but their impact on clinical events after one year is weak and inconsistent. Although unproven, the increase in mortality reported in one single trial supports a cautious approach towards the use of DES in SVG. Whether specific stent platforms, polymers or drugs are more appropriate in SVG lesions has not yet been addressed. Furthermore, research on relevant issues that are under scrutiny in native vessel subsets, like the prevalence of late stent malapposition, is required. This will certainly require cooperative efforts and the utilisation of intracoronary imaging techniques such as IVUS or optical coherence tomography.

