Abstract
Aims: A systematic review of the outcomes after drug-eluting stents (DES) implantation in saphenous vein grafts (SVGs) was performed.
Methods and results: The majority of the 33 published studies were retrospective with only two prospective randomised trials. Late loss and binary restenosis was reduced compared to bare metal stents (BMS) in all seven studies with angiographic follow-up. With the exception of one study there was no difference in mortality, myocardial infarction, or stent thrombosis between BMS and DES. The need for repeat target vessel or lesion revascularisation was lower in the DES arm in approximately half the published studies and similar in the remaining studies.
Conclusions: Until data from large, prospective, randomised-controlled studies become available, DES implantation in SVGs appears to be safe and, although not yet definitively proven, likely to reduce angiographic restenosis and the need for repeat target lesion revascularisation.
Introduction
Although drug-eluting stents (DES) provide benefit in most anatomic and clinical subsets studied, their role in the treatment of saphenous vein graft (SVG) lesions, remains controversial1-3. Most of the currently available data are retrospective and no systematic review has recently been conducted. In this manuscript we sought to summarise the published studies on the outcomes after DES implantation in SVGs.
Methods
In June 2009 we searched for studies published in English that examined the outcomes after implantation of DES in SVG lesions. Online databases (PubMed, EMBASE, Cochrane Library) and cardiology society websites (cardiosource.com, tctmd.com, crtonline.org, escardio.org) were queried using the terms drug-eluting stents, saphenous vein grafts, coronary bypass graft surgery and vein grafts. The references on the retrieved articles were also searched for additional citations. Case reports, editorials and letters were excluded. Studies that reported uncontrolled outcomes after DES implantation in SVGs and studies that compared DES vs. BMS in SVGs were included. Large DES registries that reported outcomes specifically for the subgroup of SVG lesions were also included. All articles were assessed by two reviewers (E.S.B. and B.S.) before inclusion in the review. In case of disagreement, the articles were reviewed by a third reviewer (S.B.).
Extracted data included study design, sample size, baseline characteristics, stent types, duration of follow-up, performance of routine angiographic follow-up, and outcome data. Outcomes examined were angiographic late loss and binary restenosis, mortality, myocardial infarction (MI), target-vessel revascularisation (TVR), target lesion revascularisation (TLR), and stent thrombosis.
Due to marked differences in study follow-up and in baseline patients characteristics in several of the included studies, and because most studies were retrospective, we elected to not perform a quantitative review. Instead we presented the outcomes of each study separately in summary tables.
Results
As of June 2009, 33 published studies reported outcomes after DES implantation in saphenous vein grafts (Figure 1 and Tables 1 and 2): (a) 30 retrospective studies (eight uncontrolled case series including 713 patients4-11, two studies comparing a sirolimus-eluting (SES) with a paclitaxel-eluting (PES) stent12,13, and 20 studies comparing DES patients (n=3091) with historic controls receiving BMS (n=2081)14-26; (b) one post-hoc analysis of a prospective randomised-controlled trial comparing drug-eluting with BMS that reported the outcomes for the subgroup of saphenous vein graft lesions27; and (c) two prospective randomised controlled clinical trials: one single-centre trial of a SES vs. a similar bare metal stent28,29, and one multicentre trial of a PES vs. a similar bare metal stent30.
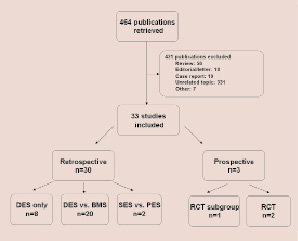
Figure 1. Outline of the study selection process. DES, drug-eluting stents; BMS, bare metal stents; SES, sirolimus-eluting stents; PES, paclitaxel-eluting stents; RCT, randomised controlled trial.
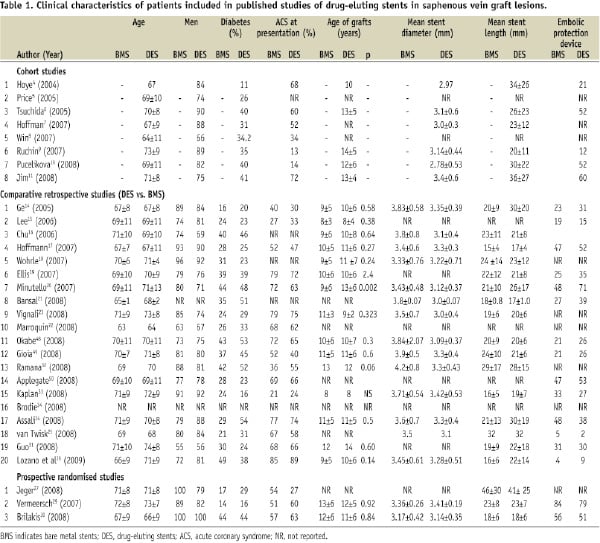
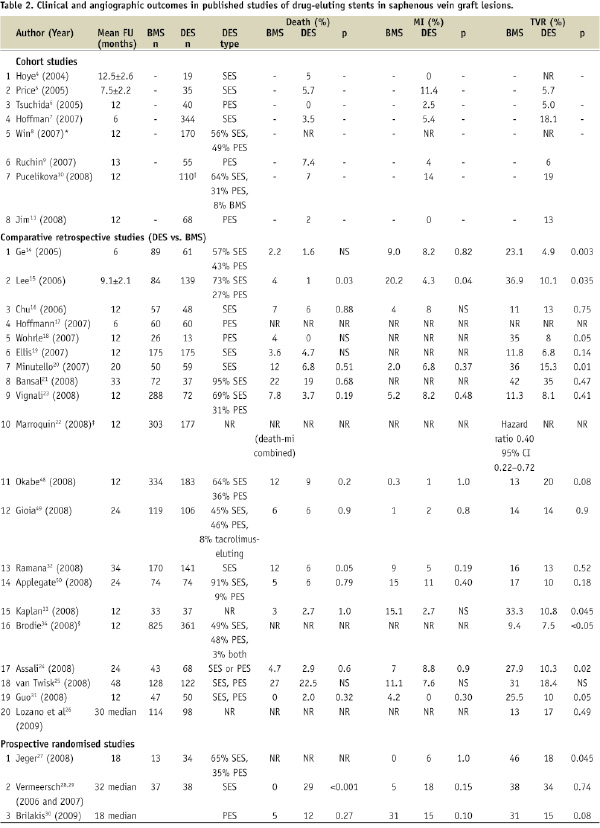
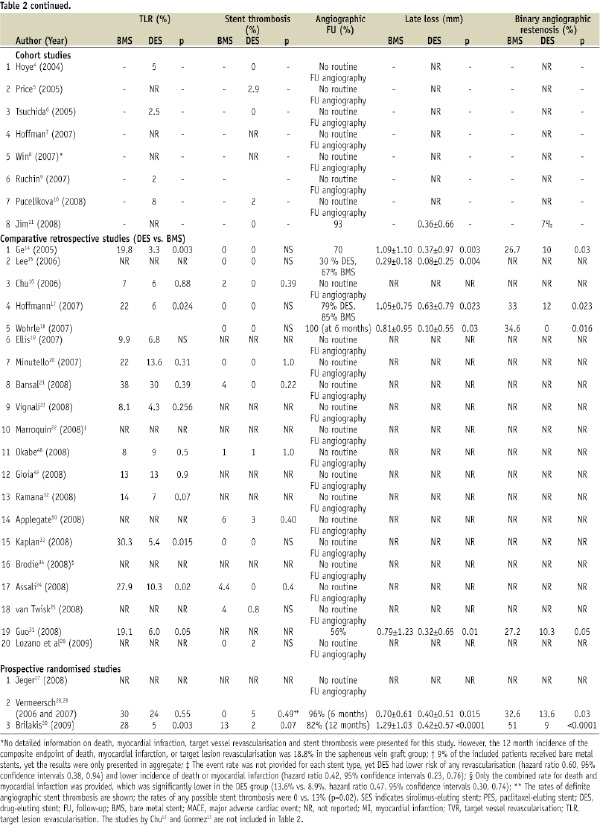
A SES or PES was used in all studies. No studies reporting outcomes after everolimus-eluting or zotarolimus-eluting stents were found. The PES used in one of the studies17 was the V-Flex Plus stent (Cook, West Lafayette, IN, USA), that is not currently in use, whereas the PES used in all other studies was the Taxus stent (Boston Scientific, Natick, MA, USA).
Patient characteristics
The clinical and angiographic characteristics of the study patients are shown in Tables 1 and 2. As is typical for patients undergoing SVG stenting, patients were old and the majority were men. Patients often had diabetes and presented with an acute coronary syndrome after a mean time of 9-13 years post coronary artery bypass graft surgery. An embolic protection device was used in a minority of patients in most studies.
Angiographic outcomes
Angiographic outcomes were reported in eight studies, including one observational cohort study11, (2008) five retrospective comparative studies14,15,17,18,31, and the two prospective randomised-controlled trials28,30 (Table 2). Follow-up angiography was done at six months14,17,18,28, or 12 months11,30,31. All seven comparative studies showed less angiographic late loss with DES implantation than with BMS. Late loss with DES ranged from 0.10 to 0.42 mm with the SES and the Taxus PES and was 0.63 mm with V-Flex Plus PES.
Clinical outcomes
Mortality was similar in the BMS and DES arm in all studies, except for two retrospective studies that showed lower mortality with DES15,32 and one prospective trial (RRISC) that showed higher late mortality with DES29 (Table 2).
The incidence of myocardial infarction was also similar between DES and BMS with the exception of one study in which MI occurred less often in the DES arm15.
Target vessel revascularisation was required less often in the DES arm of nine out of 19 retrospective studies reporting TVR14,15,18,20,22,24,31,33,34 and in the subgroup analysis of the BASKET trial27. Of the randomised trials, RRISC showed an initial reduction of TVR at six months28, which was lost during longer follow-up29, whereas SOS showed a trend for lower TVR in the DES arm (p=0.08)30.
Target lesion revascularisation was required less often in five of 14 retrospective studies reporting TLR14,17,24,31,33 and in the SOS trial30 (Table 2). The non-significant reduction of TVR in the SOS trial in spite of significant TLR reduction was due to the development of new lesions within the originally treated SVG. Similarly, Price et al showed that after SVG stenting with DES only 6% of patients required TVR, yet the incidence of major adverse cardiac events was high (35%) due to progression of disease in non-stented vessels5.
The incidence of stent thrombosis was reported in 13 retrospective comparative studies and in all three prospective studies and was similar in DES and BMS patients (Table 2).
Comparative DES studies without comparison with BMS
Two small studies retrospectively compared the outcomes after sirolimus and paclitaxel-eluting stent implantation in SVGs, and both studies showed similar outcomes. Chu compared the outcomes of 47 patients undergoing SVG stenting with a SES with the outcomes of 42 similar patients who received a PES12. At six months from implantation the incidence of major adverse cardiac events (defined as death, Q-wave MI, and TVR or TLR) was 8.5% in the SES and 10.5% in the PES group (p=0.75). Gormez et al reported similar 12-month MACE (composite of death, MI and TVR) between 46 patients undergoing SVG stenting with a PES and 25 patients undergoing SVG stenting with a SES (8.7% vs. 16%, p=0.33)13.
Discussion
Our systematic review demonstrates that in saphenous vein grafts compared to BMS, DES: (a) consistently decreased late loss and angiographic restenosis; (b) decreased target lesion revascularisation in 10 of 20 retrospective and two of three prospective studies; and (c) appear to be safe with similar rates of death (with the exception of the RRISC trial), myocardial infarction and stent thrombosis.
Saphenous vein graft lesions are among the most challenging to treat in contemporary interventional cardiology practice for several reasons: first, SVG lesions have high risk for periprocedural acute myocardial infarction; second, they have high risk for in-stent restenosis; and third, stented SVGs have high risk for developing new lesions in non-stented segments5,35. DES can not and do not address the first challenge. Our systematic review reveals that DES likely reduce the second challenge, in-stent restenosis. DES might also address the third challenge, as prophylactic stenting of moderate SVG lesions with paclitaxel-eluting stents was recently shown to prevent SVG disease progression in the “Sealing Moderate Coronary Saphenous VEin Graft LEsions With the Paclitaxel-Eluting Stent (Taxus) as a New Approach to Maintain Vein Graft Patency and Reduce Cardiac Events” (VELETI) trial36.
All seven studies that performed angiographic comparison of BMS and DES showed consistent reduction in late loss and in binary angiographic restenosis. With the exception of one study that included a polymer-free DES that is no longer in use (V-Flex Plus), all studies showed 6-12 month late loss ≤ 0.42 mm. These findings suggest that DES prevent in-stent restenosis in SVGs.
The major goal of DES is to reduce TVR and TLR. Our review shows variable results with 10 of 20 retrospective studies and two of three prospective studies showing benefit with DES and the remaining studies showing no difference between DES and BMS. There are several possible explanations for the lack of consistency among the different study findings: (1) small size of most of the studies, limiting the power to detect a difference; (2) differences in follow-up period: most of the benefit from DES is expected to occur during the first year post implantation; during prolonged follow-up, the target SVGs may fail in non-stented segments abrogating some of the benefit associated with DES use. Indeed in the SOS trial although there was significant reduction in target lesion revascularisation, there was only a trend for lower TVR, because new lesions developed in four of 41 DES patients whereas TLR was required in only two of 41 DES patients30; (3) different rates of angiographic follow-up: studies with angiographic follow-up might be more likely to demonstrate a benefit for DES in SVGs by promoting higher rates of coronary revascularisation in the bare metal stent patients, who have higher late loss and angiographic restenosis.
The incidence of death and myocardial infarction was similar in the DES and BMS groups of all studies, except one, the RRISC trial29,37. In RRISC during a median follow-up of 32 months, mortality was higher in the SES group (29% vs. 0%, p=0.001). Those findings may be due to a play of chance for the following reasons: (1) some of the deaths in the SES group were non-cardiac: of the 11 deaths in the SES group, seven were cardiac, one was due to angiographically-documented late stent thrombosis and three were sudden; (2) average annual mortality after SVG stenting is 5-7% in multiple studies38,39, therefore the expected mortality at 32 months in the BMS would be 13-19%: in RRISC the BMS group did unusually well with zero deaths, whereas the DES group did unusually poorly with 11% annual mortality; (3) no other SVG DES study with >2 year follow-up has showed such high mortality (Table 2). However, the RRISC trial findings warrant long-term follow-up in the ongoing and future SVG PCI trials, as late stent thrombosis may be an important contributor to the adverse late outcomes. Although late stent thrombosis is an infrequent event after native coronary artery stenting (annual incidence approximately 0.6%40), it may occur more frequently in the prothrombotic milieu of degenerated SVGs.
A subgroup analysis of the SOS trial demonstrated that SVG stent failure frequently presents with an acute coronary syndrome and as a complete SVG occlusion41, making prevention of SVG failure (that can likely be achieved with DES30) of paramount importance.
Our review demonstrates low utilisation of embolic protection devices in SVG PCI, in spite of their proven efficacy in reducing distal embolisation and periprocedural myocardial infarction in this setting42. Barriers to more widespread embolic protection device use, such as technical difficulty, inability to deliver and deploy the device, and cost need to be identified and addressed, especially since there are limited options to prevent no reflow (glycoprotein IIb/IIIa inhibitors have not shown benefit in most studies43 and there is limited experience with intra-graft vasodilator administration44).
Our review has several limitations. First, currently available data on use of DES in saphenous vein grafts are mostly retrospective and non-randomised. Although DES were introduced in the US clinical practice >5 years ago, only three prospective studies have examined the role of DES in SVGs: the RRISC trial28, the post-hoc analysis of the BASKET trial45, and the SOS trial30. Observational studies have several limitations, such as publication bias, tendency to overestimate treatment effects, and most importantly the unmeasured and unreported differences between the groups receiving DES or BMS in the non-randomised cohort studies. Second, most of the published studies are single-centre (except for six retrospective studies7,8,17,19,23,26 and the SOS trial30). There is no published prospective, randomised-controlled, multicentre clinical trial examining the efficacy of DES in SVG lesions using a clinical endpoint. Third, there are no published studies, with the second generation DES (zotarolimus-eluting or everolimus-eluting). Fourth, many studies did not report detailed information on the non-composite endpoints of interest (Table 2), and very few had information on the duration of dual antiplatelet therapy in the BMS and DES groups. Fifth, most published DES studies in SVGs followed the patients only for a limited period of time (as little as six months in two studies14,17). However, restenosis may occur later in saphenous vein grafts, and other complications, such as progression of non-critical SVG lesions35 and stent thrombosis may not become apparent until several months or years after implantation40,46,47. When late follow-up (median 32 months) was obtained in the RRISC trial, restenosis was similar in the DES and the BMS group29, suggesting that longer follow-up studies may be necessary to accurately assess the efficacy of DES in saphenous vein grafts. Sixth, many studies of DES in SVGs had routine angiographic follow-up (Table 2), which may overestimate the need for repeat target-vessel revascularisation (oculostenotic reflex), and the potential DES benefit.
Large, high-quality, prospective trials are needed to provide a definitive answer on the role of DES in SVG lesions. Currently three such studies are ongoing. The first is the “Prospective, Randomised Trial of Drug-Eluting Stents vs. Bare Metal Stents for the Reduction of Restenosis in Bypass Grafts” (ISAR-CABG) trial (NCT00611910), that will enrol 600 patients in two centres in Germany. Patients are being randomised to a DES arm; three DES will be used, Cypher (Cordis, Johnson & Johnson, Warren, NJ, USA), Taxus, or a local ISAR stent that is coated with rapamycin but does have a polymer) or a bare metal stent. The primary endpoint is the composite of death, MI and TLR at one year after stent implantation. The second is the “BAsel Stent Kosten Effektivitäts Trial - SAphenous Venous Graft Angioplasty Using Glycoprotein IIb/IIIa Receptor Inhibitors and Drug-Eluting Stents” (BASKETSAVAGE) (NCT00595647). BASKETSAVAGE will randomise 240 patients to a PES (Taxus Liberté, Boston Scientific, Natick, MA, USA) vs. a similar bare-metal stent (Liberté, Boston Scientific). Enrolment is occurring at several centres in Switzerland, Denmark, and Germany. The primary endpoint of the study is the composite of cardiac death, non-fatal MI and TVR. The third study is the VA Cooperative Study #571, “Drug Eluting Stents In Saphenous Vein Graft Angioplasty” (DIVA) trial. DIVA will be the first prospective, blinded multicentre clinical trial of DES vs. BMS in SVG lesions, which will utilise a clinical, rather than angiographic endpoint, will include a cost-effectiveness analysis, and will also include second generation DES. DIVA is anticipated to start enrolment during 2010.
Until data from the above trials become available, DES implantation in SVGs appears to be safe and, although not definitively proven yet, likely reduces angiographic restenosis and the need for repeat target lesion revascularisation.

