Abstract
Aims: To compare the intravascular ultrasonography (IVUS) findings between saphenous vein grafts (SVG) treated with paclitaxel-eluting stents (PES) vs. bare metal stents (BMS) in the Stenting Of Saphenous Vein Grafts (SOS) trial.
Methods and results: Of the 80 SOS trial patients, 38 had both baseline and follow-up IVUS examination and were included in this substudy: 17 patients received 28 BMS in 26 lesions and 21 patients received 30 PES in 28 lesions. Quantitative IVUS analysis was performed to determine the volume of in-stent neointimal hyperplasia (NIH) - defined as the difference between stent volume and lumen volume in the stented segments. Baseline characteristics were similar between patients who did and did not undergo baseline and follow-up IVUS. Patients receiving BMS and PES had similar stent and lumen volumes immediately after stenting. At 12-month follow-up, compared to BMS, PES-treated lesions had significantly less NIH volume (3.4 vs. 21.9 mm3, p<0.001) and neointima hyperplasia progression (1.6 vs. 17.1 mm3, p<0.001). No significant differences were seen in the 5 mm segment proximal and distal to the stent.
Conclusions: Compared to BMS, use of PES in SVG lesions is associated with significantly lower NIH formation, which may help explain the improved clinical outcomes with PES in these lesions.
Introduction
Drug-eluting stents (DES) are currently used in the majority of saphenous vein graft (SVG) interventions,1 despite limited and conflicting data on their safety and efficacy in this lesion subset2-10. The SOS (Stenting of Saphenous Vein Grafts) trial (NCT00247208) demonstrated improved angiographic and clinical outcomes with the use of paclitaxel-eluting stents (PES) compared to bare metal stents (BMS) in SVG lesions 11-14. The objective of the present study was to report the intravascular ultrasonography (IVUS) findings of the SOS study.
Methods
Patients
The SOS study design and primary results have been published11. Briefly, 80 patients undergoing SVG stenting were randomised to a PES (TAXUS; Boston Scientific, Natick, MA, USA) (n=41) or a similar BMS (Express2; Boston Scientific) (n=39). For patients with >1 lesion, the same stent type was used in all lesions. Patients were asked to return after 12 months for follow-up coronary angiography and were also followed clinically. Intravascular ultrasonography was strongly recommended (but not mandated) after initial stent deployment and at the time of follow-up angiography.
Intravascular ultrasonography
Intravascular ultrasonography imaging was performed after intracoronary administration of 100-200 mcg of nitroglycerine using motorised pullback (0.5 or 1 mm/s) with a 20 MHz or a 40 MHz commercially available scanner. The stented SVG was imaged starting at least 10 mm distal to the stent, imaging proximally to the SVG ostium. Images were recorded in CDs and were analysed at the Dallas VA Medical Centre Core Laboratory. All IVUS analyses were performed blinded to stent allocation using CAAS QIVA software (Pie Medical, Maastricht, The Netherlands).
The primary endpoint of the IVUS substudy was in-stent neointimal hyperplasia (NIH) volume, calculated as the difference between stent volume and lumen volume in the stented segments. To correct for slight differences in stent length between the baseline and follow-up IVUS examination (likely related to altered catheter movement within the stent), a NIH index was calculated as the NIH volume divided by the stent length in each IVUS pullback. Baseline and follow-up NIH indices for each stent were then multiplied by the actual known stent length to calculate a corrected NIH volume. NIH progression was calculated as the difference between the corrected NIH volume between the follow-up IVUS and the baseline IVUS.
Secondary IVUS endpoints were: 1) in-stent lumen and stent volume; 2) in-stent lumen, stent, and NIH cross-sectional areas and diameters; 3) in-stent percent area and diameter stenosis; 4) in-stent percent obstruction volume, calculated as corrected neointimal volume divided by corrected stent volume at follow up multiplied by 100; 5) incomplete stent apposition defined as greater than one stent strut separated from the vessel wall with blood speckling behind the strut; and 6) proximal and distal reference segment lumen volume, cross-sectional area and percent area and diameter stenosis, measured 5 mm from the stent border. We were unable to analyse overlapping stent segments due to small numbers (only two such lesions were included in the BMS and two lesions in the PES arm).
Statistical analysis
Statistical analyses were performed using JMP 8.0 software (SAS Institute, Cary, NC, USA). Continuous parameters are presented as mean±standard deviation or median with interquartile range and were compared using the Wilcoxon rank-sum test. Nominal parameters were presented as percentages and compared with the chi-square test. A p value <0.05 was considered statistically significant.
Results
Patients
Of the 80 patients (with 124 stents in 112 SVG lesions) enrolled in the SOS study, 38 patients (with 58 stents in 54 SVG lesions) underwent both baseline and follow-up IVUS and were included in the IVUS substudy (Figure 1). The remaining 42 patients were not included because they died prior to follow-up angiography (n=5), follow-up angiography was not performed (n=8), IVUS was not performed at the time of follow-up angiography (n=24), emergent CABG surgery was performed (n=1), or because of suboptimal IVUS image quality (n=4). The baseline characteristics of the patients who did and those who did not have follow-up IVUS were similar (data not presented), although patients with TIMI 2 flow after the initial stent procedure and those with SVGs to the right coronary artery were less likely to undergo follow-up IVUS.
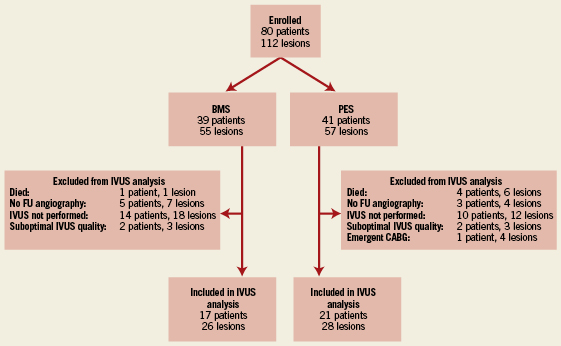
Figure 1. Flow chart describing which patients were included in the IVUS substudy of the SOS Trial.
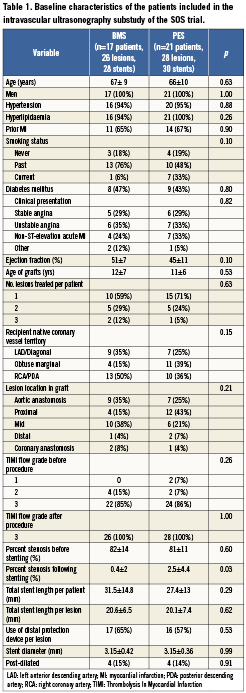
Of the 38 patients included in the IVUS substudy, 17 (with 28 stents in 26 lesions) received a BMS and 21 (with 30 stents in 28 lesions) received a PES. Both groups had similar baseline characteristics (Table 1). The average follow up time in the BMS and PES group was 306±98 days and 316±92 days, respectively (p=0.75).
Intravascular ultrasonography
The baseline IVUS measurements within the stent and in the proximal and distal 5 mm segments were similar between the BMS and PES patients (data not presented). On the follow-up IVUS, PES patients had less in-stent NIH volume, NIH area, area stenosis, NIH progression, and percent volume obstruction (Figure 2, Table 2). NIH volume <15, 15 to 30, and >30 mm3 was found in 42%, 27%, and 31% of BMS patients compared to 93%, 7%, and 0% for PES patients, respectively (Figure 3) (p=0.0002). NIH progression <3 mm3 between baseline and follow-up IVUS was seen in 15% of BMS patients vs. 61% of PES patients (p=0.0006). Follow-up IVUS measurements in the 5 mm proximal and distal to the stent were similar between the two groups (data not presented).
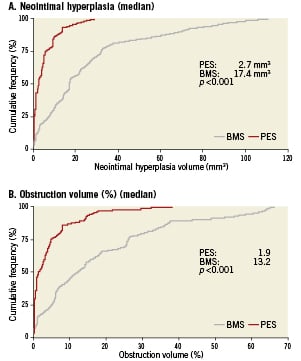
Figure 2. Cumulative frequency distribution curves of in-stent neointimal hyperplasia volume (Panel A) and percent obstruction volume (Panel B) at follow-up intravascular ultrasonography in lesions treated with paclitaxel-eluting stents (red line) or bare metal stents (grey line).
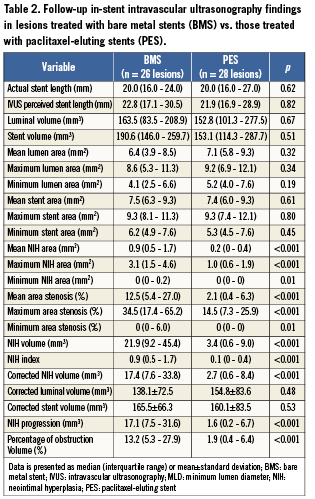
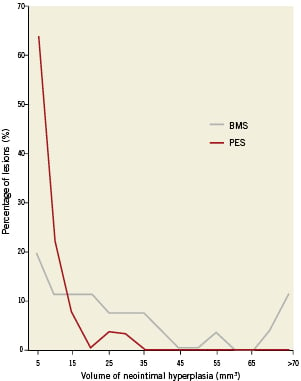
Figure 3. Distribution of neointimal hyperplasia (NIH) volume within paclitaxel-eluting stents (red) and bare metal stents (grey).
Incomplete stent apposition was documented in six lesions (11.1% of lesions treated): three with BMS (11.5%) and three with PES (10.7%) (p=0.92). In two of the BMS lesions, stent malapposition was located within the stent, while in the remaining BMS and all PES lesions the incomplete stent apposition was located at the proximal or distal edges of the stent.
Discussion
The main finding of the IVUS substudy of the SOS trial is that compared to BMS, PES-treated lesions had significantly less in-stent NIH formation, without increase in the frequency of late stent malapposition and without any difference in the measurements within 5 mm proximal and distal to the stent.
Drug-eluting stents have been shown to reduce neointimal hyperplasia formation in native coronary arteries by preventing smooth muscle cell proliferation15,16. Whether DES have similar efficacy in SVGs has been debated2, in part because SVG atherosclerosis has different pathological features compared to native coronary atherosclerosis and in part because once a SVG has a severe enough lesion to require PCI, it is likely to re-narrow or occlude elsewhere in the graft within the relatively near future. SVG atherosclerosis is usually diffuse, concentric, and friable with a poorly developed fibrous cap and little evidence of calcification, whereas native vessel plaques tend to be proximal, focal, eccentric, and non-friable with a well-developed fibrous cap and frequent calcification17. SVG atheromas tend to have more foam cells and inflammatory cells, including multinucleated giant cells, than native coronary atheromas17, express elevated inflammatory cytokine levels18, and may be more prone to thrombus formation19. Finally, the SVGs’ intimal layer has fewer cells compared to native coronary arteries20, which may have implications for ISR, that appears to be largely mediated by smooth muscle cells. Although the histologic response of SVGs to DES implantation has been poorly studied18, the response to BMS implantation is different in SVGs compared to native coronary arteries21. First, the media layer of the SVG is thinner than that of native coronary arteries and is more likely to fracture post stenting leading to aggressive neointimal proliferation. Second, there is more plaque protrusion through stent struts in SVGs, possibly predisposing to stent thrombosis. Third SVGs are more likely to form circular calcification around the stent struts. Fourth, SVGs may be more likely to have persistent inflammation around the stent struts, even long after implantation. These differences could lead to different histologic responses to DES implantation in SVGs.
A recent systematic review of published studies comparing DES and BMS in SVGs demonstrated that DES had lower angiographic late loss compared to BMS in all seven comparative studies2, five of which were retrospective comparative studies22-26, and two of which were prospective randomised-controlled trials. However, only one IVUS study of DES in SVGs has been published, the IVUS substudy of the RRISC (Reduction of Restenosis In Saphenous vein grafts with Cypher sirolimus-eluting stent) trial27,28. RRISC and SOS are the only two, randomised-controlled trial comparing DES with BMS in SVGs. In RRISC a sirolimus-eluting stent (SES, Cypher; Cordis, Warren, NJ, USA) was compared with a similar BMS in 75 patients (96 lesions in 80 SVGs); there was less angiographic restenosis at six month follow-up angiography with SES27, but significantly higher mortality after a median follow-up of 32 months29. In the RRISC trial, IVUS was performed in 59 patients (73 lesions) at six months post-stent implantation and revealed significantly less NIH volume in the SES group28 (1.3 mm3 vs. 24.5 mm3 in the BMS group, p<0.001)28. As in RRISC, the SOS trial also identified a significant reduction of NIH volume in the drug-eluting stent arm (3.4 mm3 vs. 21.9 mm3 in the BMS group, p<0.001), although follow-up angiography and IVUS was done at 12, rather than six months and different drug-eluting stents were used. A reduction of NIH with PES in SVGs was also demonstrated in native coronary arteries at nine months in the TAXUS IV trial16 (18 mm3 with PES vs. 41 mm3 with BMS, p<0.0001). It is of interest that both absolute and relative effects of PES on suppression of NIH appear to be greater in SVG than native coronary arteries. This may be due to the smaller smooth muscle cell layer in SVG, or a differential injury response.
Our study findings have important implications. First, they suggest that PES effectively suppress neointima formation in SVGs, similar to native coronary arteries, supporting the recently proposed use of undersized DES in SVG lesions to decrease the risk of periprocedural myocardial infarciton30. Second, although the number of patients in the IVUS substudy of SOS was small, PES appeared to be safe with no increase in edge stenosis (“candy wrapper” phenomenon), as was seen in the Taxus IV trial16. However, greater suppression of NIH in SVGs compared to native coronary arteries could result in incomplete stent strut coverage. This, in turn, could lead to increased risk for DES thrombosis in SVGs, that could explain the adverse long-term outcomes observed in the RRISC trial29. Novel, high resolution intracoronary imaging modalities, such as optical coherence tomography, may soon provide improved insights on DES strut coverage in SVGs19.
Our study has important limitations. The SOS protocol strongly encouraged (but did not mandate) intravascular ultrasonography (IVUS) after initial stent implantation and during follow-up angiography. Accordingly, only approximately half of the SOS study patients had good quality baseline and follow-up IVUS and were included in the IVUS substudy; however the characteristics of the patients who were and those who were not included were similar. Many patients (especially in the BMS group) had occluded SVGs at follow-up12 and therefore follow-up IVUS could not be performed; this would bias the results in favour of BMS, yet PES were still found to have significantly less NIH. Therefore, the reduction in NIH with PES may well be greater than what we report. Finally, NIH is a surrogate endpoint; its suppression with DES may not necessarily translate into improved clinical outcomes in SVGs, as was demonstrated in the RRISC trial. However, PES were associated with improved clinical outcomes in SOS11, as well as the Moderate VEin Graft LEsion Stenting With the TAXUS Stent and Intravascular Ultrasound (VELETI) Pilot trial31, the ARRIVE (TAXUS Peri-Approval Registry: A Multicentre Safety Surveillance) registries32 and the recently published Is Drug-Eluting Stenting Associated With Improved Results in Coronary Artery Bypass Grafts (ISAR-CABG) trial.33
In summary, PES significantly reduced NIH formation in SVG lesions compared to bare metal stents in the SOS trial with no increase in late malapposition, supporting their superior efficacy in this challenging lesion subset.
Funding
The SOS trial was funded by the Department of Veterans Affairs VISN-17 Startup Award and by the Clark R. Gregg grant of the Harris Methodist Foundation to Dr. Brilakis.
Conflict of interest statement
O. Jeroudi received support from NIHLBI grant T35-HL086346. J. de Lemos has received speaker honoraria from Bristol-Myers Squibb/Sanofi-Aventis and consulting income from Johnson and Johnson (<$10,000). O. Obel works predominantly with cardiac rhythm devices and has speaker agreements with St. Jude, Medtronic, and Boston Scientific. T. Addo has served on the Speakers Bureau for Sanofi-Aventis, Merck-Schering, Eli Lilly and Daiichi-Sankyo. J. Rossen participated in multicentre clinical studies supported by Boston Scientific. P. Berger owns equity in Lumen, Inc. S. Banerjee has served on the Speakers’ Bureau for St. Jude Medical Center, Medtronic Corp., and Johnson & Johnson and has received a research grant from Boston Scientific. E. Brilakis has received speaker honoraria from St. Jude Medical and Terumo, research support from Abbott Vascular and Infraredx, and his spouse is an employee of Medtronic. The other authors have no conflicts of interest to declare.

