Abstract
Aims: Our aim was to compare the outcomes of complete revascularisation (CR) and incomplete revascularisation (IR) in multivessel coronary artery disease (CAD), with and without intravascular ultrasound (IVUS) guidance, in the drug-eluting stent (DES) era.
Methods and results: Overall, 2,132 consecutive patients with multivessel CAD, defined as at least two epicardial vessels with >70% stenosis, had at least one DES implant. Chronic total occlusions were not analysed. Successful treatment of epicardial vessels and significant branches was termed CR; otherwise, treatment was defined as IR. CR and IR were further categorised according to the use of IVUS. The primary outcome was death or Q-wave myocardial infarction (QWMI). Secondary outcomes included the rates of non-QWMI and repeat revascularisation, the latter assessed as either target vessel revascularisation (TVR) or target lesion revascularisation (TLR) at one year. CR was associated with lower rates of death/QWMI (HR 0.66 [0.4-0.9]; p=0.048) and non-QWMI at one year (1.1% vs. 2.6%; p=0.017). Completeness of revascularisation was not independently associated with repeat intervention, but rates of both TVR (89% vs. 93%; p<0.001) and TLR (91% vs. 95%; p<0.001) were higher with CR than IR. IVUS decreased the rates of TLR irrespective of completeness of revascularisation (p-interaction=0.75).
Conclusions: CR in selected patients gives better outcomes than IR in multivessel CAD at one year. IVUS guidance can further improve results by reducing rates of repeat intervention irrespective of completeness of revascularisation.
Abbreviations
CAD: coronary artery disease
CR: complete revascularisation
CTOs: chronic total occlusions
IR: incomplete revascularisation
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Current opinion on multivessel coronary artery disease treatment attributes the magnitude of survival benefit to the level of complete revascularisation (CR) achieved1. Historically, percutaneous coronary intervention (PCI) achieves lower rates of CR compared to surgery2,3. This may partially explain the poorer outcomes in patients undergoing multivessel PCI compared to coronary artery bypass surgery4 and has led to the recommendation of surgery over PCI for severe multivessel disease5.
However, incomplete revascularisation (IR) is a surrogate marker of a sicker population with more anatomical complexity6. This complicates comparisons between CR and IR, as apparent differences in survival may be at least partially due to the different populations involved. Indeed, in the current era, the use of arterial anastomosis has been shown to be more important than the degree of revascularisation completeness3,4,7. On the other hand, even when the rate of CR by PCI approaches that obtained by surgery4, PCI patients still experience worse outcomes, with a particularly high incidence of repeat revascularisation4 due to a combination of restenosis, residual CAD, and atherosclerosis progression.
Although drug-eluting stents (DES) have markedly reduced the restenosis risk, approximately 10% of patients undergo unplanned repeat procedures during the first year8. Of these, half are due to restenosis and half are a result of atherosclerosis progression or previously unrecognised significant lesions left untreated8. Intravascular ultrasound (IVUS) improves outcome by allowing both procedure optimisation9 and also, in some cases, by detecting severe lesions not seen by angiography10, resulting in a more CR. However, the impact of IVUS on the completeness of revascularisation is unknown.
Therefore, our aims were: 1) to assess the independent effect of CR on death and myocardial infarction (MI) in the DES era; and 2) to evaluate the impact of IVUS on outcome according to completeness of revascularisation.
Methods
STUDY POPULATION
This was a retrospective study of 2,132 consecutive patients with multivessel disease who underwent PCI at a single centre between 2003 and 2013. Multivessel disease was defined as the presence of at least two major epicardial vessels with ≥70% diameter stenosis. For inclusion in the study, participants were required to have at least one DES implant. We excluded patients with: 1) past CABG; 2) ongoing MI requiring primary PCI; 3) cardiogenic shock; or 4) true chronic total occlusions (CTOs), defined as an epicardial vessel with a de novo lesion with Thrombolysis In Myocardial Infarction flow grade 0 and proven duration ≥3 months11.
REVASCULARISATION CATEGORIES
Our database was queried for multivessel patients, who were then categorised as either CR or IR (CR: individuals with successful treatment of all major epicardial lesions, including side branches ≥2.5 mm in vessel diameter, at a single stage, or patients who achieved CR through staged procedures within eight weeks of the index intervention; IR: individuals with any epicardial vessel or side branch left untreated). Additionally, patients were categorised according to the IVUS guidance. Figure 1 shows a flow chart of our research, which was approved by the local Institutional Review Board.
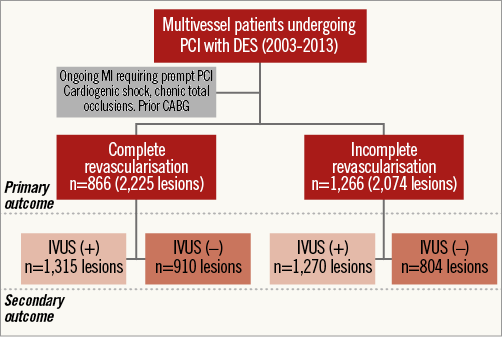
Figure 1. Study flow chart.
DEFINITIONS
We defined baseline risk factors as follows: arterial hypertension – blood pressure >140/90 mmHg or the use of antihypertensive therapy; hypercholesterolaemia – fasting cholesterol >250 mg/dl or the use of lipid-lowering therapy; diabetes mellitus – serum blood glucose levels >126 mg/dl or by the use of either oral hypoglycaemic medications or insulin; chronic renal failure (CRF) – serum creatinine >2.0 mg/dl; congestive heart failure (CHF) – defined as evidence of fluid retention from cardiac causes. Peripheral vascular disease was defined as any vascular disease affecting non-coronary arteries.
OUTCOMES
All deaths were considered cardiac unless otherwise documented. MI was defined as an elevation in creatine kinase-MB of twice the upper limit (2.6 ng/ml). MI was further categorised as Q-wave MI (QWMI) if new Q-waves >1 mm occurred in ≥two contiguous leads; otherwise, non-QWMI was diagnosed. Repeat revascularisation due to recurrent ischaemia either by CABG or PCI was classified as target lesion revascularisation (TLR), where the stented segment or the 5 mm proximal and distal borders required intervention, or target vessel revascularisation (TVR) when intervention occurred in remote vessel segments. Stent thrombosis (ST) was defined according to the Academic Research Consortium criteria as definite or probable.
PROCEDURE AND ADJUNCTIVE THERAPIES
PCI was performed using standard techniques with all patients treated with aspirin and thienopyridines, in particular clopidogrel, before the procedure. Anticoagulation included either heparin, with a bolus dose of 60 U/kg and additional dose(s) to achieve an activated clotting time of 200-300 s, or bivalirudin 0.75 mg/kg followed by an infusion of 1.75 mg/kg/hr during the procedure. The choice of DES type was left to physician discretion. Routine IVUS measurements were recorded and interpreted by each physician assisted by a dedicated technician. The Atlantis® S or SR Pro (Boston Scientific, Marlborough, MA, USA) or the Eagle Eye® catheter (Volcano Corp., Rancho Cordova, CA, USA) was used. Angiographic success was defined as the presence of Thrombolysis In Myocardial Infarction 3 flow and angiographic lesion final diameter <30%.
DATA COLLECTION AND CLINICAL FOLLOW-UP
Patients’ demographics, risk factors, baseline characteristics, and procedural details were prospectively recorded in a dedicated system controlled by independent personnel. Outcomes of interest were assessed through telephone interview, office visits, chart review, and a social security linkage process at 30 days, six months and one year. All outcomes were systematically adjudicated by interventional cardiologists blinded to the study objectives.
STUDY OBJECTIVES
The primary objective was to determine whether CR by PCI is independently associated with a reduction in the composite of death/QWMI. The secondary objectives were the impact of completeness of revascularisation on repeat intervention at one year, and the impact of IVUS guidance on outcomes according to the completeness of revascularisation.
STATISTICAL ANALYSIS
Binary variables are reported by counts and percentages while the continuous variables are presented as mean±standard deviation. Differences were tested by either chi-square or Fisher’s exact test, as appropriate. Continuous variables were tested by parametric or non-parametric tests where data were non-normally distributed. We first assessed the time-to-survival for the endpoints of death/QWMI and repeat revascularisation by the Kaplan-Meier method and by the log-rank test. Secondly, the independent effect of CR on outcomes was assessed by a univariate model followed by a multivariable Cox proportional hazards model, entering variables with p<0.10. Subgroups of interest included diabetics, age >80 years, and CHF. Finally, we tested the interactions of IVUS guidance with each outcome. All analyses were conducted using SAS software, version 9.2 (SAS Institute, Cary, NC, USA).
Results
BASELINE CLINICAL AND ANGIOGRAPHIC CHARACTERISTICS ACCORDING TO COMPLETENESS OF REVASCULARISATION
The study consisted of 2,132 multivessel CAD patients undergoing PCI with at least one DES implanted. Of these 1,312 (2,403 lesions) received only DES. Overall, approximately 25% of the patients had a history of MI and 36% had diabetes. CR was achieved in 40.6% of the population. IR patients were older, more frequently female, and were also characterised by a higher prevalence of comorbidities such as CRF, CHF, and peripheral vascular disease compared to CR patients (Table 1).
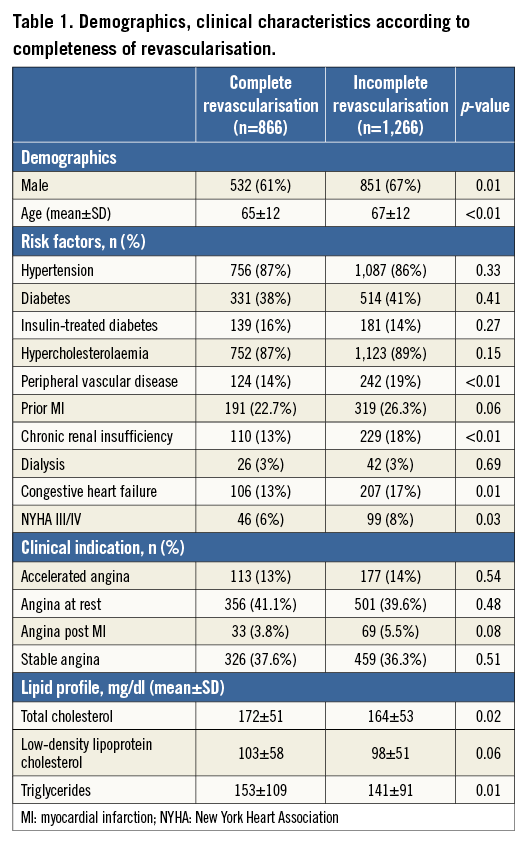
Table 2 depicts the angiographic characteristics according to completeness of revascularisation. The IR group had more diseased vessels and more anatomical complexity, even after excluding true CTOs, as shown by a higher prevalence of type C lesions. Indeed, IR patients more often required lesion preparation through rotational atherectomy (3.9% vs. 2.1%; p<0.001) and were less likely to undergo direct stenting compared to CR patients (32% vs. 44%; p<0.001). On the other hand, CR patients required more extensive intervention, resulting in more lesions being dilated and more stents per patient, totalling an average 12 mm greater stent length compared to IR patients (43±23 mm vs. 31±19 mm; p<0.001).
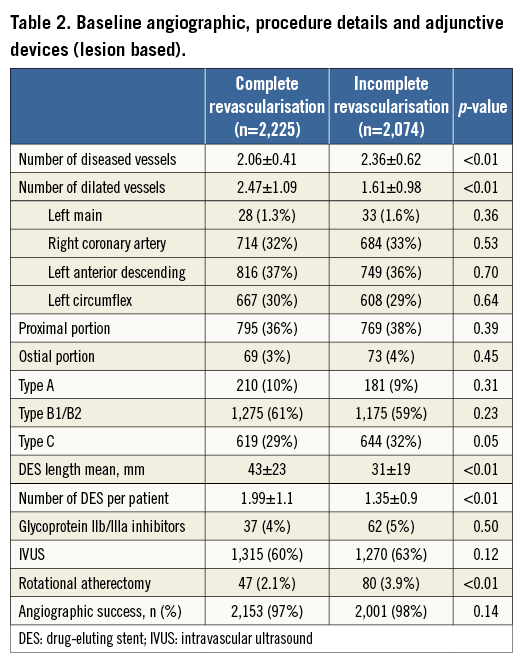
DEATH/QWMI ACCORDING TO COMPLETENESS OF REVASCULARISATION
Overall, death/QWMI occurred in 143 (6.7%) patients at one year. Individuals who underwent CR were less likely to die or experience QWMI compared to IR patients, with a difference noted at six months (4.0% vs. 6.1%; p=0.038), which had widened by one year (5.0% vs. 7.9%; p=0.008) (Table 3). This difference was mostly driven by a reduction in all-cause mortality at six months (3.7% vs. 5.7%; p=0.036) and at one-year follow-up (4.6% vs. 7.5%; p=0.009). Nonetheless, there was a reduction of non-QWMI in the CR group at one year (1.1% vs. 2.6%; p=0.017).
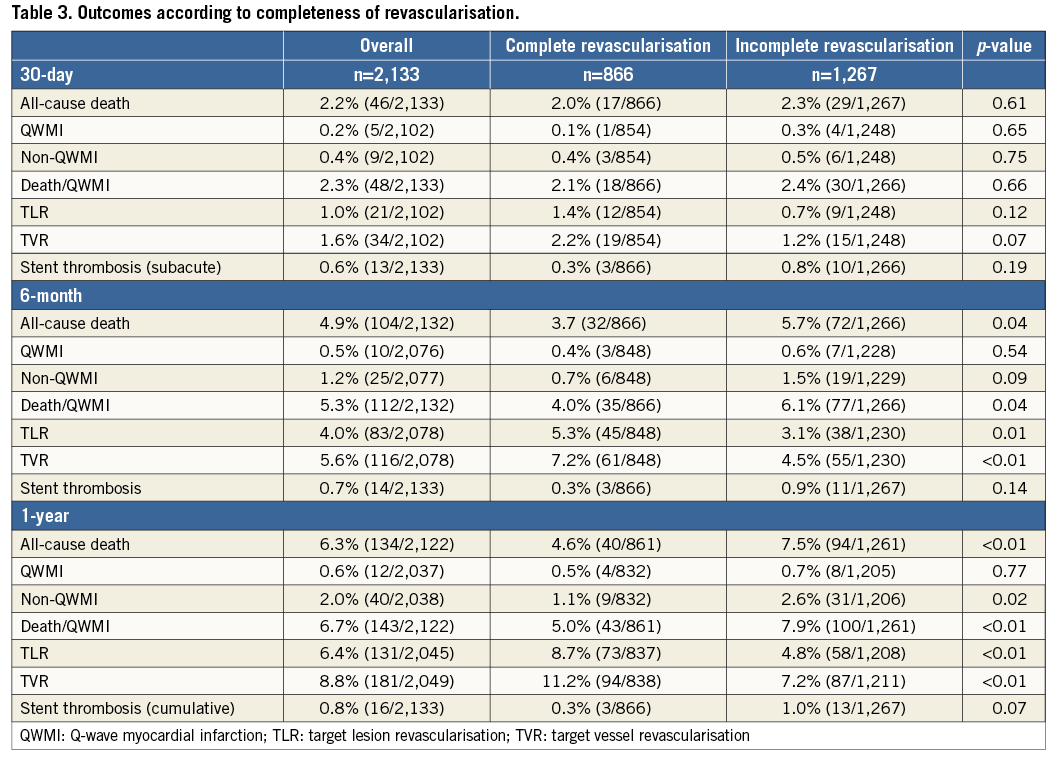
There was an early separation in survival curves of death/QWMI between the groups resulting in a 37% risk reduction favouring CR at one year (HR 0.63 [0.44-0.90]; p=0.011) (Figure 2). After adjusting for confounders, CR remained independently associated with lower rates of death/QWMI compared to IR (HR 0.66 [0.38-0.99]; p=0.046) (Figure 3). Notably, CR benefit was consistently in the same direction across the pre-specified high-risk subgroups (age >80 years, diabetes and CHF), and no significant interaction was found for these pre-specified subgroups.
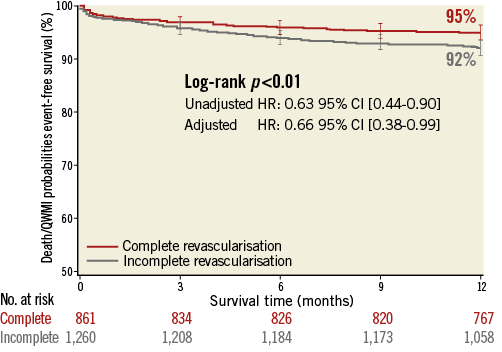
Figure 2. Time-to-death/QWMI survival at one year according to completeness of revascularisation.
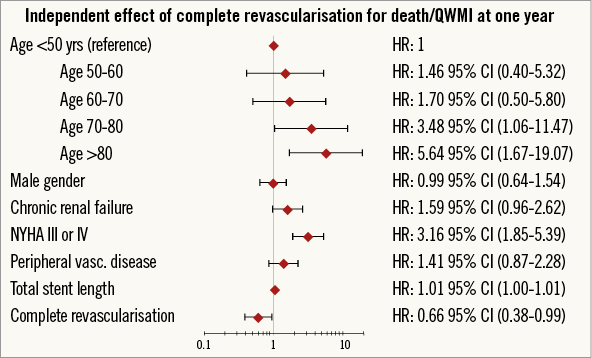
Figure 3. Forest plot correlates for death/QWMI at one year.
REPEAT INTERVENTION AND STENT THROMBOSIS ACCORDING TO COMPLETENESS OF REVASCULARISATION
CR patients required higher rates of repeat revascularisation at one year at both vessel and lesion levels (TVR: 11.2% vs. 7.2%; p=0.002; and TLR: 8.7% vs. 4.8%; p<0.001) (Table 3). This resulted in an approximately 1.9-fold higher risk of TLR in CR compared to IR patients (HR 1.88 [1.33-2.66]; p<0.001) (Figure 4). Given the inclusion of successful PCI cases, CABG was required in 0.6% of patients and was similarly distributed between the CR and IR groups (0.5% vs. 0.7%; p=0.457). Conversely, TLR by means of PCI was more frequently reported in CR than in IR patients (8.3% vs. 4.1%; p<0.001).
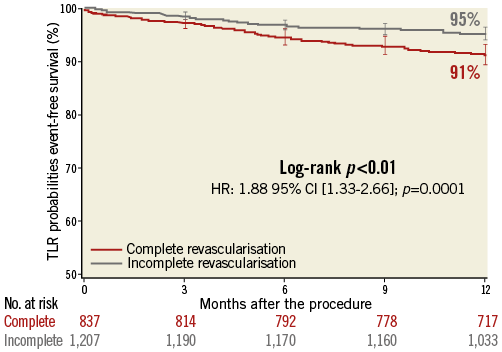
Figure 4. Time-to-TLR survival at one year according to completeness of revascularisation.
Importantly, when we considered patients treated with only DES, this difference was no longer statistically significant for TVR (6.4% vs. 6.8%; p=0.777) or TLR (4.7% vs. 4.3%; p=0.764). These analyses were confirmed after adjustment where the effect of completeness of revascularisation on repeat intervention was no longer statistically significant for TVR (HR 1.28 [0.90-1.83]; p=0.172) and for TLR (HR 1.52 [0.99-2.33]; p=0.06) (Figure 5A, Figure 5B). Strikingly, the strongest protective factor for both TVR and TLR was revascularisation using only DES.
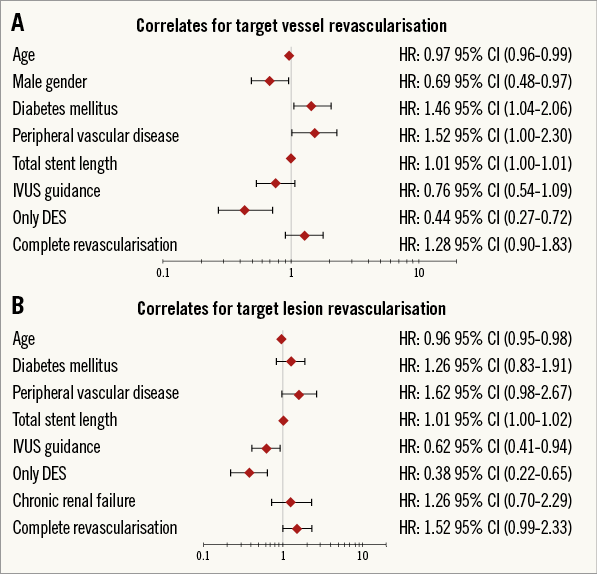
Figure 5. Forest plot correlates for repeat intervention. A) Target vessel revascularisation. B) Target lesion revascularisation.
The rates of stent thrombosis were numerically lower in CR at all time points, i.e., at 30 days (0.3% vs. 0.8%; p=0.197), six months (0.3% vs. 0.9%; p=0.143), and one year (0.3% vs. 1.0%; p=0.074).
IMPACT OF IVUS GUIDANCE ON OUTCOMES ACCORDING TO COMPLETENESS OF REVASCULARISATION
The use of IVUS guidance was similar between CR and IR groups, with 1,315 and 1,270 lesions assessed, respectively (p=0.12). Using univariate analysis, IVUS guidance did not reach statistical significance for association with death/QWMI (HR 0.82 [0.58-1.16]; p=0.26). On the other hand, IVUS was associated with a lower rate of repeat intervention in both CR and IR groups (Figure 6). CR patients with IVUS guidance had an increased survival time free from TLR and a trend for TVR (90.2% vs. 86.3%; p=0.096) at one year which was also observed in IR patients. After adjusting for confounders, IVUS guidance was independently associated with a reduction in TLR and with a trend for TVR (HR 0.76 [0.54-1.09]; p=0.135). Additionally, the reduction of TLR rate observed with IVUS guidance was apparent regardless of the completeness of revascularisation (p-interaction=0.75).
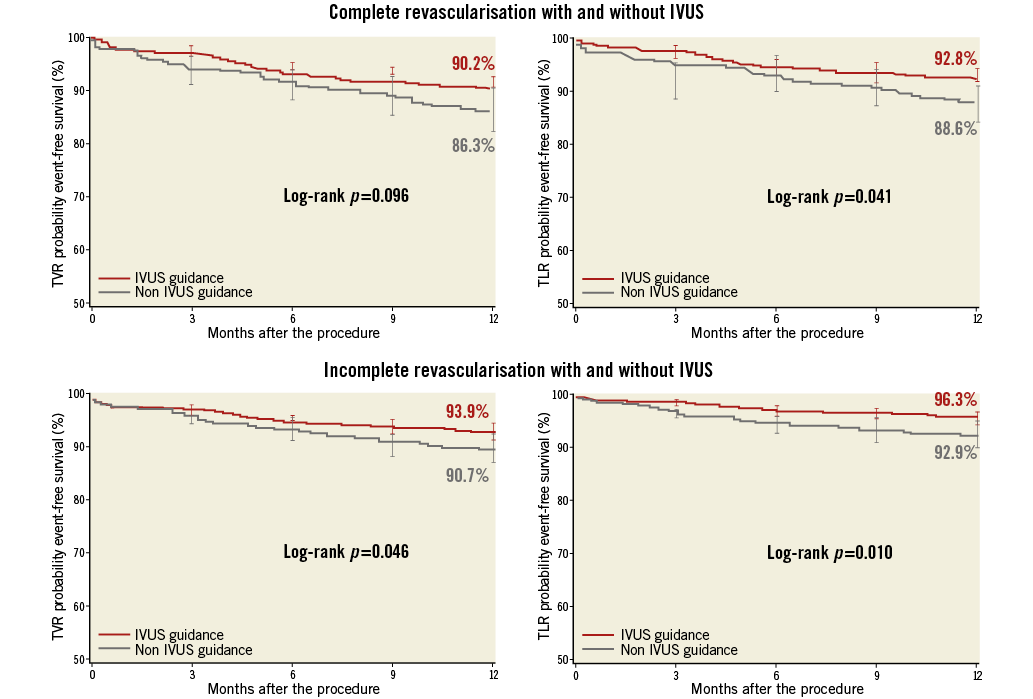
Figure 6. Time-to-repeat intervention with and without intravascular ultrasound.
Discussion
This study is the first to assess the impact of IVUS guidance on outcomes according to completeness of revascularisation in the DES era. Our main findings are: 1) CR is associated with reduced rates of death/QWMI; 2) IVUS is associated with reduced rates of repeat procedures regardless of revascularisation completeness.
In this large series of 2,132 multivessel patients undergoing PCI, CR was achieved in 41% of cases, resulting in a 34% risk reduction for death/QWMI compared to IR. Additionally, non-QWMI was ≈58% less likely to ensue during follow-up when a CR strategy was initially undertaken. Our findings are in keeping with the general belief that CR portends better outcomes. Nonetheless, our study differs from others. First, all participants received at least one DES, with 1,312 of those being entirely treated with first- or second-generation devices. Second, 2,585 vessels had IVUS assessment assigned, apportioned between CR and IR groups.
In general, studies that demonstrated poor survival following IR by surgery or PCI have marked clinical and angiographic differences between CR and IR groups12. Conversely, where a more neutral effect on outcome was reported2,7,13,14, fewer differences were observed between CR and IR groups. This brings into question whether survival outcome depends on degree of revascularisation at all. Attempting to take the differences between CR and IR groups into account in such studies is challenging15.
CTOs remain the strongest predictor of IR in the contemporary PCI era6. Notably, in the SYNTAX trial, the success rate for CTO attempts by means of PCI was no more than 44% in those in whom clinical equipoise was deemed by either strategy. Thus, it is intuitive that these patients would have higher residual disease with large areas of ischaemic myocardium, increasing the risk of a future event16. Additionally, such failed attempts may further worsen myocardial ischaemia, thus magnifying the occurrence of events in IR11. Therefore, the excess of hazard attributed to IR PCI patients may, in part, be related to these biases. Our study circumvents some of these disparities by excluding true CTOs, making CR and IR groups anatomically more balanced.
Residual CAD has been validated as a determinant of poor outcomes for low-, moderate-, and high-risk CAD patients6,17,18, regardless of presentation or revascularisation approach. Notably, in a sub-analysis from the COURAGE trial, plaque burden was a better predictor of events than ischaemia19, while the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) trial showed that non-culprit plaque severity correlates with unstable morphology findings20. Therefore, the combination of a higher angiographic cut-off (≥70% diameter stenosis) along with the high IVUS assessment rate in our study may have led to a “true” anatomical CR by the treatment of unanticipated lesions, explaining the benefit we found in CR patients in relation to death and MI.
We found that CR had higher crude rates of TLR and TVR compared to IR at one year. This contrasts with studies showing equivalent13 or higher rates of repeat interventions in IR patients2,3,21,22. CR patients who undergo more extensive revascularisation receive more stents than IR patients, resulting therefore in higher rates of restenosis. Although this was confirmed in our study, after adjustment, completeness of revascularisation was no longer independently associated with TVR or TLR. Moreover, IVUS reduced the rates of repeat intervention in both groups.
Overall, the preference for IVUS-guided PCI at our centre contributed to the remarkably low TVR and TLR rates observed, compared to other studies2,6,21. Furthermore, the benefit of IVUS was apparent irrespective of the completeness of revascularisation. We speculate that this consistent reduction in repeat intervention in both groups indicates that IVUS may also be linked to uncovering significant lesions unanticipated by angiography, along with target lesion optimisation. Indeed, this may contribute to explaining the persistent disparity in outcome according to completeness of revascularisation after PCI in contrast to surgery. Although the SYNTAX trial showed similar rates of death and MI comparing surgery and PCI in patients with less complex disease, the incidence of repeat intervention was significantly higher across all SYNTAX score tertiles4.
The reasons for repeat intervention after multivessel PCI are not explained solely by device failure. Indeed, only half of the unplanned procedures after PCI in the DES era are related to TLR, whereas the other contingent occurs at non-target sites, representing unrecognised lesions8. These findings can explain, in part, the persistently higher rates of repeat revascularisation after multivessel PCI compared to surgery and also the lower impact of CR after surgery compared to PCI. Therefore, multivessel PCI can be further improved both by identifying ischaemic lesions at non-target sites and by optimising implantation techniques at the target sites. Our study indicates that IVUS consistently reduced the rates of repeat revascularisation at target and non-target domains. Another factor contributing to the low rates of repeat revascularisation is the high frequency of DES in our study. Strikingly, PCI only with DES was the strongest protective factor, with a 62% and 56% risk reduction for TVR and TLR, respectively.
Interestingly, a trend towards a lower rate of cumulative ST was noted in CR patients, despite a 10 mm higher mean stent length (p=0.074). This agrees with the SYNTAX trial6, which documented a cluster of ST among patients with IR who received fewer stents. The reasons for this are unclear, but might include more calcified lesions, diffuse disease, and underexpanded stenting. In our study, the equivalent angiographic success rate, the higher rate of IVUS guidance, and the similar post-diameter stenosis in CR and IR patients make stent optimisation an unlikely cause for the increase in ST in the IR patients. This question therefore requires additional research.
Limitations
Our study has several limitations. The first is the retrospective design, where the decision on whether to perform CR or IR was not controlled. Therefore, we cannot exclude residual selection bias which might have affected our results. Thus, our conclusions should be interpreted as hypothesis-generating. Second, our database is not audited. However, this is one of the largest, single-centre registries to evaluate revascularisation completeness in the DES era. Although the exclusion of true CTOs can be viewed as a limitation, PCI of CTOs remains infrequently performed in the USA. Therefore, this is unlikely to change our results since our preferred revascularisation approach is surgery23. Fourth, although the secondary prevention medications of the CR and IR groups at hospital discharge were comparable, we could not assess differences between the groups in compliance during follow-up.
Conclusions
CR determines better outcomes than IR for multivessel disease in the DES era. IVUS guidance further improves outcomes by reducing the rates of repeat intervention irrespective of completeness of revascularisation. Our study suggests that multivessel PCI with DES assisted by IVUS is a viable strategy for multivessel CAD patients.
| Impact on daily practice Percutaneous treatment of multivessel CAD patients in the DES era remains challenging. Although there is still scope for device and technique refinements, residual and unrecognised plaque burden may play an important role in determining outcome, in particular the need for repeat revascularisation. The residual CAD burden is frequently underestimated by angiography, which may partially explain the impact of completeness of revascularisation in patients undergoing PCI. In contrast, adjunctive use of intravascular imaging in PCI refines the CAD assessment, leading to procedure optimisation and better outcomes irrespective of the completeness of revascularisation. Therefore, intravascular imaging has the potential to improve PCI results, further narrowing the performance gap between surgery and PCI in multivessel patients. |
Conflict of interest statement
R. Waksman reports personal fees from Biotronik, personal fees from Medtronic, grants and personal fees from AstraZeneca, grants and personal fees from Boston Scientific, personal fees and grants from Biosensors International, personal fees from Abbott Vascular, grants from The Medicines Company, and grants from Edwards Lifesciences. The other authors have no conflicts of interest to declare.

