Abstract
Aims: This study aimed to examine the strategy of hybrid percutaneous coronary intervention (PCI) –bare metal stent (BMS) and drug-eluting stent (DES)– versus exclusive DES implantation for patients undergoing multivessel PCI.
Methods and results: A cohort of 2,065 patients who underwent PCI (698 hybrid, 1,367 exclusive DES) were followed clinically up to one year. The primary outcome was target vessel revascularisation-major adverse cardiac events (TVR-MACE). Patients presenting with cardiogenic shock, anaemia (haematocrit <25), and bypass graft PCI were excluded. Only patients with ≥2 stents in two different lesions were analysed for this study. Baseline and procedural characteristics were similar. Major in-hospital complications and subacute stent thrombosis rates were similar. At one year, there was no difference in TVR-MACE (hybrid 17.2% vs. DES 14.6%, p=0.128). On multivariable analysis, hybrid PCI was not a predictor of TVR-MACE. The strongest predictors of TVR-MACE at one year were hypertension and African American race. Cumulative stent thrombosis rates at one year were similar in both groups.
Conclusions: Patients who undergo hybrid PCI have similar composite in-hospital and 1-year outcomes as those who undergo exclusive DES PCI. The hybrid stent approach should be considered for patients with multivessel PCI since it can lower the procedure cost without increasing adverse events.
Introduction
Drug-eluting stents (DES) have reduced the need for repeat revascularisation when compared to bare metal stents (BMS) after percutaneous coronary intervention (PCI).1,2 Implantation of a BMS, however, may be more cost effective than implantation of a DES. Given the recent heightened concern for late stent thrombosis and death, there is a resurgence of BMS use, which has reached a plateau. There have been attempts to find new strategies of revascularisation to minimise cost without the expense of an increase in adverse events. However, when faced with the dilemma of more complex subsets of patients and lesions, there are no large clinical trials to help guide physicians. Faced with this uncertainty, physicians have presented novel approaches to multivessel PCI.
One approach to consider is implantation of both a BMS and DES during multivessel PCI (hybrid PCI). Hybrid PCI may be preferred by operators because of the desire to adhere to “on-label” Food and Drug Administration (FDA) indications for DES, economic considerations, operator preference for certain lesion and patient subsets, and concern for future adverse events (stent thrombosis).3-6 There are few data regarding outcomes after a hybrid PCI. The current study was undertaken to report a large “real world” experience in patients who had hybrid PCI versus exclusive DES PCI for multivessel coronary artery disease. Clinical outcomes were compared. Our aim was to determine whether the hybrid PCI approach would reduce cost without increasing clinical adverse events.
Methods
Patient population
A cohort of 2,065 patients (698 with hybrid PCI, 1,367 with exclusive DES) who underwent PCI from April 2003 to October 2007 at our institution were studied and followed clinically up to one year. Clinical events were recorded and compared between the two groups. Patients with cardiogenic shock, severe anaemia (haematocrit <25), and all bypass graft PCI were excluded from this analysis. After we obtained approval for a Health Insurance Portability and Accountability Act waiver from our local Institutional Review Board, we extracted from the entire database all patients who corresponded to the inclusion criteria and conducted a retrospective analysis of the clinical outcomes. In both groups, implantation of ≥2 stents in two different native coronary lesions was required. The decision to implant a BMS or a DES was at the individual operator’s discretion.
Procedure
Coronary angioplasty was performed in the conventional manner and coronary stents or other procedures/devices were used when required. Adjunct balloon inflation was added after initial stent deployment in some cases. Optimal stent implantation was carefully monitored using an iterative technique with intravascular ultrasound monitoring in the majority of cases. In all cases, the interventional strategy, including the use of direct stenting, pre- or post-dilatation, intravascular ultrasound, use of ablative devices, choice of periprocedural adjunctive antiplatelet therapy, and choice of antithrombotic regimen was at the discretion of the responsible physician. Angiographic success was defined as a stenosis of ≤30% with a Thrombolysis in Myocardial Infarction (TIMI) flow grade 3. All patients received aspirin 325 mg/day before the intervention and continued this regimen indefinitely. Additional antiplatelet therapy with either clopidogrel 75 mg/day (after a loading dose of 300 to 600 mg) or ticlopidine 250 mg twice daily was instituted in all patients and was continued for ≥1 year.
The anticoagulation regimen consisted of either bivalirudin with or without low-dose heparin mixed in the contrast media or weight-adjusted unfractionated heparin. Following sheath insertion, bivalirudin was administered as a bolus of 0.75 mg/kg and then as an intravenous infusion of 1.75 mg/kg/hour for the duration of the procedure to achieve an activated clotting time between 250 and 300 seconds for unfractionated heparin and above 250 seconds for bivalirudin. Activated clotting time was routinely measured before and during the PCI (Hemochron; International Technidyne, Edison, NJ, USA). The use of IIb/IIIa inhibitors was at the discretion of the operator.
Study definitions
The study end points for analysis were in-hospital complications (death, myocardial infarction [MI], and coronary artery bypass grafting [CABG]) and major adverse cardiac events (MACE) at one year, defined as a composite of death, MI, and target vessel revascularisation (TVR). A dedicated data-coordinating centre (Data Center, MedStar Research Institute, Washington, DC, USA) performed all data management and analyses. Pre-specified clinical and laboratory data during hospitalisation periods were obtained from hospital charts reviewed by independent research personnel who were blinded to the objectives of the study. Death was defined as all causes of mortality. MI this admission is defined as creatine kinase-MB elevation ≥2 times the upper limit of normal and/or the presence of ischaemic electrocardiogram changes including new Q-waves or ST elevation ≥1 mm. ST-segment elevation MI was defined as chest pain with ≥1 mm of ST-segment elevation on ≥2 contiguous electrocardiogram leads, chest pain refractory to medical therapy with associated ST-segment depression in leads V2 to V5 (consistent with posterior injury), or a new left bundle branch block. Cardiogenic shock was defined as maximal systolic pressure <90 mmHg for ≥30 minutes unless treated with inotropes, or pump failure as manifested by a cardiac index <2.2 liter/minute per m2 and pulmonary capillary wedge pressure >18 mmHg and/or persistence of hypotension or pump failure after correction of contributing extra-myocardial factors (e.g., hypovolemia, hypoxemia or acidosis) and/or peripheral signs of hypoperfusion (e.g., peripheral vasoconstriction, urine output <30 ml/hour or altered sensorium). Left ventricular ejection fraction (LVEF) was measured by echocardiography or angiography during the hospitalisation. Type C lesions (American College of Cardiology/American Heart Association [ACC/AHA] classification) are defined as diffuse (>2 cm), excessive tortuosity of proximal segment, >90˚ angulation, chronic total occlusions and/or bridging collaterals, inability to protect major side branches, and degenerated vein grafts with friable lesions. FDA-approved indications for DES are defined as de novo lesions that are 28-30 mm in length and 2.5-3.75 mm in diameter. DES in our analysis included only paclitaxel- and sirolimus-eluting stents. Target lesion revascularisation (TLR) and TVR were characterised by repeat percutaneous or surgical intervention of the treated lesion or vessel, respectively, and were clinically driven. All clinical events were adjudicated by source documentation by independent physicians who were not involved in the procedures. Stent thrombosis is considered “definite” as defined by the Academic Research Consortium, which states, “the presence of acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion.”
Statistical analysis
Statistical analysis was performed using the Statistical Analysis System version 9.1 (SAS Institute Inc., Cary, NC, USA). Data are expressed as mean ±SD for continuous variables and as percentages for categorical variables. Student’s t test was used to compare continuous variables and the chi-square test or Fischer’s exact test was used to compare categorical variables. A p value <0.05 was considered to indicate statistical significance. Cox proportional hazard analysis was used to identify predictors of MACE. Cox proportional regression models were used to control for differences in the groups. Comparisons were made between hybrid PCI and exclusive DES groups.
Results
Baseline characteristics
Baseline and procedural characteristics were similar. (Table 1) The hybrid group had more acute MI and history of congestive heart failure (CHF). The exclusive DES group had more African Americans, hypertension, hyperlipidaemia, left anterior descending PCI, and angiographic success.
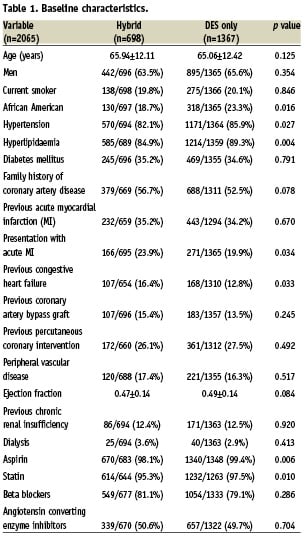
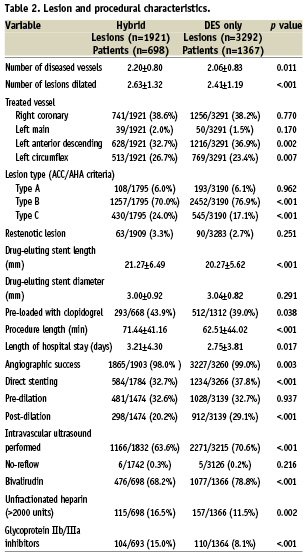
Lesion and procedural characteristics
BMS compromised approximately 53.3% (949/1,780) of the hybrid PCI group. The average number of DES implanted per patient was 1.45±0.81 for the hybrid group and 2.40±0.66 for the DES only group (p <.001). The hybrid PCI group had a higher number of diseased vessels, more type C ACC classification lesions, longer DES stent lengths, and had longer procedure lengths and hospital stays. (Table 2) Bivalirudin was more likely to be used in the DES only group whereas heparin and glycoprotein IIb/IIIa inhibitors were more likely to be used in the hybrid PCI group.
In-hospital outcomes
Major in-hospital complications defined as death, MI, and CABG were similar in both groups. (Table 3) Subgroup analysis revealed in-hospital death was higher in the hybrid PCI group, possibly reflecting a sicker population at presentation. MI on admission (which was significantly higher in the hybrid group) was a strong predictor of in-hospital death (OR: 3.34 [1.51-7.39], p=0.003). Major bleeding was similar in both groups, however overall vascular complications were higher in the hybrid PCI group, which may be a reflection of the greater use of heparin and glycoprotein IIb/IIIa inhibitors. There were no differences in subacute stent thrombosis or no-reflow phenomenon between the groups.
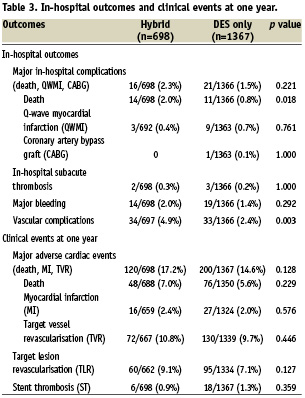
One-year outcomes
MACE, defined as death, Q-wave MI, and TVR at one year were similar in both groups. (Table 3) Subgroup analysis did not reveal any differences in death, MI, TVR, and TLR. In the univariable model, independent predictors of MACE included African American race, history of MI or CHF, dialysis, MI on admission, LVEF, number of diseased vessels, length of hospital stay, acute renal failure, and type C lesions. On multivariable analysis, the strongest predictors of MACE at 1 year were African American race (HR: 1.4 [1.0-1.8], p=0.021), history of CHF (HR: 1.4 [1.0-1.9], p=0.041), hypertension (HR: 1.8 [1.2-2.8], p=0.007), left anterior descending artery disease (HR: 1.3 [1.0-1.6], p=0.038). Intravascular ultrasound was a negative predictor of MACE (HR: 0.70 [0.54-0.91], p= 0.007). Hybrid PCI was not an independent predictor of MACE at one year even after adjustment for differences in baseline characteristics. When defining MACE as death, Q-wave MI and TLR, there was still no difference between the two groups even after multivariate analysis (HR: 1.24, [0.95-1.64], p=0.119). Rates of cumulative stent thrombosis at one year were similar between the groups.
Discussion
Our study demonstrates that hybrid PCI for multivessel coronary artery disease is safe and has a similar MACE rates at one year compared to the exclusive DES group. On subgroup analysis, there was no difference in death, MI, TVR, TLR, or stent thrombosis at one year between the two groups. Hybrid PCI patients were sicker at presentation. After adjustment for baseline characteristics, hybrid PCI was not an independent predictor of MACE at one year. In-hospital complications were similar between the two groups. In-hospital death was higher in the hybrid PCI group, possibly reflecting a sicker population at presentation. There was no difference in subacute stent thrombosis or in the no re-flow phenomenon.
Multivessel coronary artery disease requires careful planning and strategy prior to revascularisation factoring in co-morbid conditions, LV function, future operations, probability of future adverse events, ability to comply with dual antiplatelet therapy, and patient’s goals. We previously published a report showing that multivessel PCI with DES in non-diabetics has equivalent major adverse cardiac and cerebrovascular event rates compared to CABG.7 To our knowledge, however, there are no large comparisons between hybrid PCI and exclusive DES strategies.
In multivessel PCI, patient and lesion characteristics, compliance, economic considerations, as well as the need for- and risk of prolonged dual antiplatelet therapy should be factored into the decision making process prior to performing a multivessel PCI. For example, patient characteristics considered independent predictors of MACE in our study included hypertension, African American race, history of CHF, and LAD disease. In these patients, a DES may be preferred in certain lesion subsets compared to other populations, although this needs to be examined in a randomised study. The use of IVUS was a negative predictor of MACE. The decision to use a BMS or DES should be independent of whether an additional DES implantation is planned.
The FDA-approved indications for the DES have guided clinicians although adherence is low. FDA indications for DES include the following: “Sirolimus-eluting stents should be used in discrete de novo lesions of ≤30 mm in length and reference vessel diameters of ≥2.5 mm-≤3.5 mm; Paclitaxel-eluting stents should be used in discrete de novo lesions of ≤28 mm and reference vessel diameters of ≥2.5 mm-≤3.75 mm.” Lesion characteristics for which to implant a BMS include long lesions, large or small diameter vessels, bifurcation lesions, restenotic lesions, left main lesions, ostial lesions, totally occluded lesions and operator preference. All of our patients had multivessel disease that required ≥2 stents (at least one being DES). We excluded previously bypassed patients because of the uncertainty of DES in saphenous vein graft (SVG) PCI and the possibility of an increase in adverse events compared to “de novo” lesions. In our study, the decision to use a BMS was left to the operator.
Controversies do exist regarding which lesions may be best treated with DES. Mirabella et al showed that implanting a DES for complex lesions (type B2/C ACC classification lesions) and BMS for simple lesions (type A/B1 lesions) may be an acceptable approach.8 There was a reduction in TLR in patients who had type B2/C lesions and received a DES compared to BMS, while there was no difference seen in type A/B1 lesions. This finding is inconsistent with other studies, which showed that complex lesions typically are not suitable for DES implantation and may have more adverse events. Identification of lesion class by ACC/AHA lesion classification may assist in choice of stent type (BMS versus DES) however, large randomised trials need to determine if it is solely the classification, or if other factors are also involved. The Society of Coronary Angiography and Intervention (SCAI) classification simplifies lesion classification and may better predict success and complications, but it is unknown if this would assist in choosing a BMS or a DES.9 In our study, type C lesions accounted for a significantly higher percentage of hybrid PCI versus exclusive DES PCI although type C lesion was not a predictor of MACE in our study.
In our study, the hybrid strategy had similar MACE rates, including restenosis rates. Although this finding is conflicting, there are numerous studies that show increased adverse events with DES implantation in complex lesions. In one observational study, the adjusted outcomes did not differ between DES and BMS, although there was a significantly lower rate of repeat revascularisation in the DES group.10 Subsets of lesion and patient characteristics for which DES were considered included patients with in-stent restenosis of BMS, left main stenosis, acute MI, diabetes, total occlusions, and small diameter vessels.11-18 However, these studies are small and do not have long-term follow up, thus leaving the issue unresolved. It has been argued that improved techniques of PCI, including high pressure inflations, intravascular ultrasound guidance, and prolonged dual antiplatelet therapy, have substantially reduced the risk of stent thrombosis and possibly justify the use of DES for more complex lesions; however this has not been adequately studied. In a report by Varani et al, patients undergoing multivessel PCI were divided into three groups (BMS only, DES only, and mixed).19 In that study, there were no differences in 1-year outcomes among the three groups when DES were used in high-risk patients and lesions. They also reported that the use of ≥1 DES reduced the risk of TVR by 37% and MACE by 29%. Other unresolved issues include duration of dual antiplatelet therapy in patients with hybrid PCI. Since there appears to be a significant association between unapproved use of DES and stent thrombosis, duration of prolonged dual antiplatelet therapy is still uncertain in these circumstances.
Hybrid PCI approach would result in a significant reduction in cost to the patient and the health care system compared to the exclusive DES approach. In 2003, according to manufacturer data of DES, Medicare reimbursements for a DES in the in-patient hospital setting were anywhere from $1,700-$2,600.20 DES cost about three times as much as BMS. According to the AHA 2009 update, there were 1,313,000 PCIs done in 2006.21 On the other hand, the FDA and multiple registries approximate that 50-60% of PCI with DES is for unapproved indications. This would translate to a cost savings of approximately $1 billion per year if the appropriate PCI strategy was employed. Reduction in duration of prolonged dual antiplatelet therapy could also account for additional cost savings to the health care system. One report found that when calculating cost analysis and taking into account late stent thrombosis, there was little cost per quality-adjusted life-year gained and cost per revascularisation avoided when using DES.22 It has been proposed by the ACC/AHA guidelines that only patients with the highest risk of restenosis should be considered for a DES because of the cost to the health care system.23
There are limitations to our study. Our study was a retrospective analysis with its inherent limitations. We excluded SVG PCI, although this would only have further impacted favourably towards a hybrid PCI approach since this is also an indication to avoid DES. Our analysis did not include newer DES, although there is still only limited comprehensive long-term data for second-generation DES. In addition, we could not analyse the reason for implantation of a BMS. Long- term (>1 year) outcomes must also be studied before definitive conclusions about hybrid PCI can be made, especially because of the risk of DES very late stent thrombosis. Nevertheless, we have demonstrated that hybrid PCI reduces cost and has similar adverse events as an exclusive DES approach in patients undergoing multivessel PCI.
In conclusion, our study confirms that a hybrid PCI approach for multivessel coronary artery disease reduces cost without increasing adverse events at one year. Multivessel PCI with the hybrid PCI strategy should be utilised in contemporary clinical practice and would substantially reduce the cost burden to the health care system without an increased risk to the patient.

