Abstract
Aims: To evaluate the long-term effectiveness and cost-efficacy of drug-eluting stents (DES) in a real world setting of multivessel percutaneous coronary intervention (PCI).
Methods and results: We evaluated the 2-year outcome of all multivessel PCI in de novo lesions enrolled in a prospective web-based multicentre registry from July 2003 to December 2006. Among the 2,898 eligible patients, 1,315 were treated with bare-metal stent (BMS) alone, 657 with DES alone, and 926 with both. At 2-years, use of DES was associated with a lower propensity score adjusted incidence of major adverse cardiac events (MACE), death and myocardial infarction, and target vessel revascularisation (TVR) compared with BMS but only in patients at high risk of TVR. No difference was apparent between «pure» DES and the mixed approach. The matched cost-effectiveness analysis revealed DES to be more costly and more effective with a reasonable incremental cost-efficacy ratio for any MACE avoided only in patients with a high risk of TVR and only in comparison with «pure» BMS patients.
Conclusions: In this real-world multivessel PCI registry, the use of DES and a mixed approach were associated with a 2-year reduction of adverse clinical outcomes in comparison with BMS especially in patients with a high risk of TVR. DES were cost-effective only in patients at high risk of TVR.
Introduction
The introduction of drug-eluting stents (DES) in clinical practice has led to a significant improvement of multivessel PCI (percutaneous coronary intervention) medium-term results almost approaching those of coronary artery by-pass graft (CABG), due to the reduction of new revascularisation rates in comparison to bare-metal stents (BMS)1-7. In more recent clinical trials comparing multivessel PCI and CABG, all lesions were treated with DES5-7. However, it is not clear if it is really mandatory to implant DES in all lesions of patients referred for multivessel PCI.
Safety issues about multiple DES utilisation are based on the higher incidence of subacute and late stent thrombosis observed in patients treated with DES-PCI versus BMS-PCI8. It is of note that stent thrombosis is strongly related to the total stent length and number of DES implanted in meta-analysis of randomised clinical trials (RCTs)9.
Moreover, there is an issue related to incremental costs of such a strategy especially in resource limited health care systems, without a clear documentation of superiority in comparison to a more selective DES use.
The mixed approach (MIX) with DES and BMS utilisation in different lesions in the same patient is quite diffused in everyday practice (estimated prevalence in 11-13% of all PCI procedures)10,11, but not specifically investigated because it is excluded as a group in all registries and RCTs evaluations.
The aim of this study was to evaluate DES utilisation modality in multivessel PCI in a large real-world registry, the crude and adjusted 24 months clinical results comprehensive of angiographic stent thrombosis, and the cost-effectiveness analysis in this setting of three different approaches (BMS only, DES only and MIX).
Methods
Population
The Registro AngiopLastiche Emilia-Romagna (REAL) is a multicentre web-based registry established in a northern Italy region (Emilia-Romagna) prospectively collecting clinical, angiographic and procedural data of all PCI performed in the 13 regional catheterisation laboratories. The REAL was launched in July 2002 under the coordination of the Health Care and Social Regional Agency with the aim of monitoring DES diffusion and utilisation and to evaluate their impact on clinical practice. Follow-up is available through several regional databases (mortality registry, hospital discharge records, outpatient clinic database).
The registry, already described in detail elsewhere12,13 has the distinctive characteristic to pursue a selective utilisation of DES following the recommendations given by the regional cardiologic and cardiac surgical commission at the time of their introduction in clinical practice.
In this study, we considered patients treated with multivessel PCI (at least one stent in two distinct territories) enrolled from July 2003 until December 2006, with the exclusion of patients not resident in the Emilia-Romagna region, those with acute myocardial infarction, shock, left main trunk treatment, previous PCI or CABG. The study complies with the Declaration of Helsinki.
Procedure
All patients gave written informed consent prior of PCI procedure. Coronary angiography has been performed according to standard practice and the decision on type of revascularisation (complete or incomplete), type of stents (DES or BMS), glycoprotein GPIIbIIIa inhibitors utilisation, a single or a staged procedure was left to the discretion of the operators. Multivessel PCI was performed in a single session in most of the cases and in 14% in a staged fashion.
BMS used were stainless steel (Libertè, Boston Scientific, Natick, MA, USA) or chromium-cobalt alloy (Vision, Abbott Vascular, Abbott Laboratories, IL, USA; Driver, Medtronic Vascular, Santa Rosa, CA, USA; Skylor, Invatec, Brescia, Italy) stents; DES were sirolimus eluting stent (Cypher™; Johnson & Johnson, Miami, FL, USA) and paclitaxel eluting stent (Taxus™, Boston Scientific, Natick, MA, USA). Before procedure all patients received aspirin ≥100 mg and clopidogrel 300 mg or ticlopidine 250 mg x 2 (at least for three days). After PCI patients were maintained on lifelong aspirin and on clopidogrel for at least one month for those without acute coronary syndrome (ACS) who were treated with a BMS and for 6-12 months for those with unstable angina/non-Q wave myocardial infarction treated with BMS14 or for those treated with at least one DES.
Endpoint
Follow-up data, including vital status, at 12 and 24 months were independently obtained from the Emilia-Romagna Regional Health Care Agency, which has direct access to municipal registries (for death certificates) and hospital discharge records. This warranted a complete follow-up for all patients resident in the region. The prospectively collected data from the web-based registry regarding all repeat surgical/percutaneous interventions performed during follow-up were matched with the administrative data to identify any inconsistencies. Specific queries were sent to the individual institutions to justify/correct discrepancies between the data recorded on the web-based registry (compiled by interventional cardiologists) and the administrative data (largely provided by independent cardiologists). Hospital records were reviewed for additional information whenever deemed necessary.
Total and cardiac death, non-fatal acute myocardial infarction (AMI), target vessel revascularisation (TVR), cumulative major adverse clinical events (MACE), angiographic stent thrombosis, were the examined events. AMI during follow-up resulted from code SDO 410.x1 defining hospital admission for a new myocardial infarction, excluding post-procedural myocardial necroses (Q wave MI when .x was different from .7 or non-Q wave MI when .x = .7). TVR was defined as any re-intervention (surgical or percutaneous) to treat a stenosis in the same coronary vessel treated at the index procedure, within and beyond the lesion limits. Re-intervention following scheduled angiographic control (non-clinically driven) were excluded. Cumulative MACEs included death, myocardial infarction or TVR. Angiographic stent thrombosis was defined as a complete occlusion or a flow-limiting thrombus in a previously treated artery. Lesion length and vessel reference diameter were estimated visually by the operators.
Cost analysis
Given that comparisons were made among patients treated in a similar percutaneous fashion, we assumed that care, comprehensive of length of hospital stay, in-hospital and post-discharge therapy, and non-invasive assessment at follow-up, was alike in all three considered groups. So, only differential costs were taken into account considering standard costs calculated on disease related group (DRG)’s reimbursement, corrected by the type and number of stents, costs of follow-up events (standardised by DRG) and those items (clopidogrel treatment) having a different behaviour in the three groups.
Initial costs Cost of the index procedure derived from: DRG of balloon PCI (DRG 518 – 5,425 Euros), corrected at 25% with DRG 516 (PCI and AMI – 7,433 Euros), considering that >50% of patients had an ACS and almost 25% a non- Q-wave AMI, plus cost of all the implanted stents.
Mean stent price had progressively decreased during the three and a half years of the study period, from 600 to 400 Euros for BMS, and from 1,800 to 1,450 Euros for DES.
Staged procedures costs were the sum of single procedures costs.
Follow-up costs Pharmacological treatment costs were calculated as follows: clopidogrel accounted for a 2 Euros daily cost, considering the most recent standard of care (one month for patients treated with BMS without ACS, and 12 months for patients treated with BMS associated with ACS and for those treated with at least one DES).
Death: no adjunctive costs if not associated to hospital admission for AMI or TVR; TVR-PCI without AMI: DRG 517 (5,946 Euros); TVR-PCI with Q or non-Q-wave AMI: DRG 516 (7,433 Euros); TVR-CABG: DRG 107, comprehensive of coronary angiography pre-CABG (18,447 Euros); Q or non- Q-wave AMI without TVR: DRG 122 (4,599 Euros).
Twelve and 24 months costs Mean 12 and 24 months costs were calculated adding to initial costs, those of follow-up events and of clopidogrel therapy in all groups.
Statistical analysis
Continuous variables were expressed as mean ±SD and were compared using Student’s unpaired t-test. Categorical variables were expressed as counts and percentages and chi-square test was used for comparison. The cumulative incidence of adverse events was estimated according to the Kaplan-Meier and compared by the log rank test. Because of the observed differences in baseline characteristics between the treatment groups, three separate propensity score analyses were carried out by use of a logistic regression model15: DES versus BMS, DES versus MIX and MIX versus BMS. This analysis included a number of clinical, angiographic and procedural variables, such as age, sex, diabetes, prior myocardial infarction, neo-plastic disease, chronic renal disease, heart failure, peripheral and cerebrovascular disease, ACS at admission, left ventricular ejection fraction <35%, warfarin therapy, left anterior descending artery, number of lesions treated, reference vessel diameter, lesion length, ostial lesion, chronic total occlusion, bifurcation, year and hospital of treatment. The logistic models by which the three propensity score were estimated showed good predictive value (C-statistic= 0.838 DES versus BMS, 0.729 DES versus MIX, 0.774 MIX versus BMS) and calibration characteristics by the Hosmer-Lemeshow test (p= 0.409 DES versus BMS, 0.651 DES versus MIX, 0.724 MIX versus BMS). The score was then incorporated into subsequent proportional hazards models as a covariate.
The same model was applied to compare the clinical outcome of the treatments within subgroups of patients: high and low risk for restenosis. For this purpose we initially performed a multivariable logistic regression analysis to identify the independent predictors of target vessel revascularisation at 1-year in patients treated with BMS only. The variables, selected as p<0.10, were: male sex, diabetes, proximal left anterior descending, proximal left circumflex, total lesion length, reference vessel diameter. From the logit estimated by logistic regression analysis we obtained the function of likelihood that is calculated by the following formula:
p=elogit(p)/(1+elogit(p)) with logit (p) = b0+b1*x1+b2*x2+...+bn*xn
This formula allowed us to assign also to DES and MIX-treated patients the same baseline «probability» (i.e., one year TVR risk). Subsequently, the entire population was subdivided into quintiles of risk, in order to identify the relative efficacy of the three treatments within each quintile of risk. Since statistically significant differences between the rate of TVR within two years were found only in the fourth and fifth quintile, patients were divided into two subgroups: «low risk» (first, second and third quintile) and «high risk» (fourth and fifth quintile).
All analyses were performed with the SAS 9.1 system.
Economic analysis
Comparison of one and two years total events and total costs among the three groups were made in propensity score matched subgroups. The matching was done utilising a propensity score calculated with the variables previously described. Each patient was paired with the lowest difference in propensity score match15. If one patient presented more than one exact match or more matches with the same difference, the selection was random. Three hundred and seventy-four (374) couples of patients were analysed in the DES versus BMS comparison at one year and 244 at two years, 582 couples in the MIX versus BMS at one year and 395 at two years, 441 couples of patients in the DES versus MIX at one year and 216 at two years.
The incremental cost-effectiveness of DES was estimated in terms of a ratio between the difference in costs and the difference in the number of MACE events: three different analysis were conducted according to the couples (DES versus BMS, DES versus MIX, MIX versus BMS) analysed. Results were expressed as an incremental cost-effectiveness ratio (ICER), indicating the cost for one MACE event avoided. Given the relatively short time period considered, costs and benefit were not discounted.
Two group comparisons were done with two sample t-test (with unequal variances after testing this assumption), Wilcoxon signrank test and distributional assumptions were assessed using the one-sample Kolmogorov Smirnov test. Non-parametric bootstrapping was used to estimate differences in average costs and time to events, using 5,000 bootstrap samples drawn from the original dataset16. Probabilities of a strategy being cost effective were calculated in relation to willingness to pay for one MACE avoided, and presented through a cost effectiveness acceptability curve17.
Subgroup analyses were carried out considering patients at low and high risk of TVR, as previously described. All the analyses were performed with SAS 9.2 system.
Results
There were 26,800 PCI procedures in the period between July 2003 – December 2006, of which 20,400 patients were resident of the Emilia-Romagna region; 5,400 (26%) were performed in at least two different vessels and 4,700 with at least two stents implanted. After exclusion of patients with AMI, left main stem treatment or previous revascularisation procedures, 2,898 multivessel PCI procedures were obtained: 1,315 (45%) with BMS only, 657 (23%) with DES only, and 926 (32%) with both BMS and DES. Clinical and procedural characteristics of patients in the three groups are summarised in Table 1.
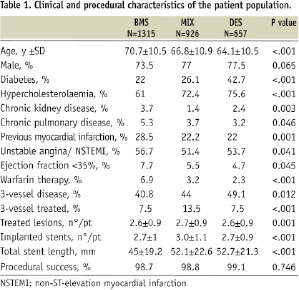
Patients treated with BMS were older, had a higher frequency of chronic renal disease, chronic obstructive pulmonary disease, previous MI, reduced left ventricular ejection fraction, ongoing warfarin therapy, while DES recipients were more often diabetic, hypercholesterolaemic and with a higher prevalence of three vessel disease. The MIX group had a higher frequency of three vessel PCI, higher number of lesions treated and more stents implanted. The total stent length was superior in the DES and MIX groups in respect to BMS group. Procedural success was high and equivalent in the three groups.
Mean lesion length increased progressively from BMS, to MIX and DES group, while mean reference vessel diameter decreased from BMS, to MIX and DES group (Table 2).
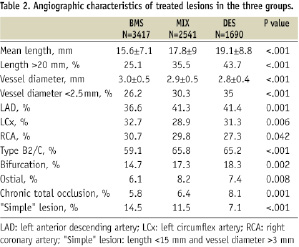
Left anterior descending, B2/C and bifurcation lesions were treated more often in DES and MIX groups.
Lesions treated with DES were again longer, in smaller vessels, more complex, more often in LAD, ostial and chronically occluded in the mixed group, while those treated with BMS in the same group were simple in 21% of cases.
Median follow-up was 920 days. Figure 1 shows the unadjusted event rates in the three groups.
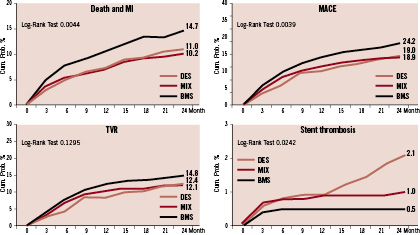
Figure 1. Unadjusted 2-year clinical event rate in the three groups.
We observed a higher two year total mortality in the BMS group (p<0.001), and the cardiac death was BMS 5.2%, MIX 3.6%, DES 2.8%, p=0.028. Incidence of AMI was similar in the three groups (p=0.272); 50% of total AMI were associated to a TVR PCI. Almost 60% of AMI were non-Q wave MI (at two years BMS 5.2%, MIX 3.2%, DES 4.4%), while the rate of Q wave MI was 2.1% in BMS, 2.8% in MIX and 3.1% in DES group (p=0.539); out of total 86 Q wave MI during the follow-up, 43 (50%) were followed by TVR and 11 (13%) by cardiac death within 30 days.
TVR were slightly more frequent in the BMS group, without statistical significance, while two years crude incidence of MACE was lower in the DES and MIX groups. Definite two years angiographic stent thrombosis was significantly increased and characteristically presented also after one year in the DES group.
In patients with events during the follow-up period, multiple adverse events (AMI and TVR) were more frequent in the DES group; for this reason the mean number of events per patient increased in DES group from 1-year (BMS 0.26, MIX 0.18, DES 0.17) to 2-years of follow-up (BMS 0.34, MIX 0.26, DES 0.31).
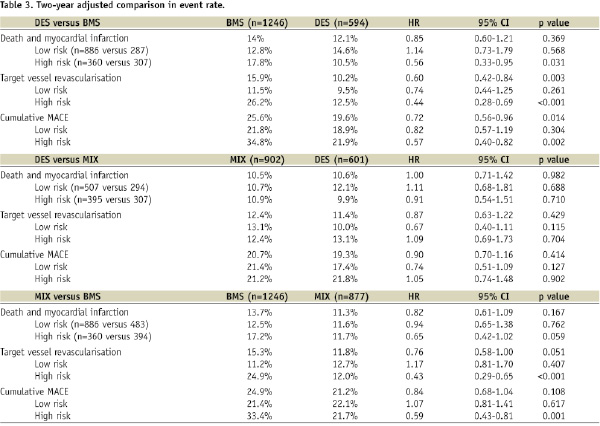
To adjust for differences in the baseline clinical and angiographic characteristics, a propensity score analysis of the data was performed in the whole groups and in the subgroups at low and high risk of TVR, as described in the methods, and previously published18. The adjusted comparison between DES and BMS resulted in lower combined death and MI rate in high risk patients treated with DES and lower TVR rate prevalently driven by the high risk subgroup (Table 3), whereas there were not differences in event rates in low risk patients. DES did not show any significant advantage over MIX approach neither in the general population nor in any risk subgroup. As compared to BMS, mixed approach was associated to a not statistically significant reduction of hard events (AMI and death) and to a significant reduction of TVR only in high risk subjects.
The mean initial, follow-up and total costs at 12 and 24 months in the three groups are shown in Table 4.

The cost-efficacy comparisons between matched groups with the incremental cost-efficacy ratio (ICER) for avoided MACE are summarised in Table 5.
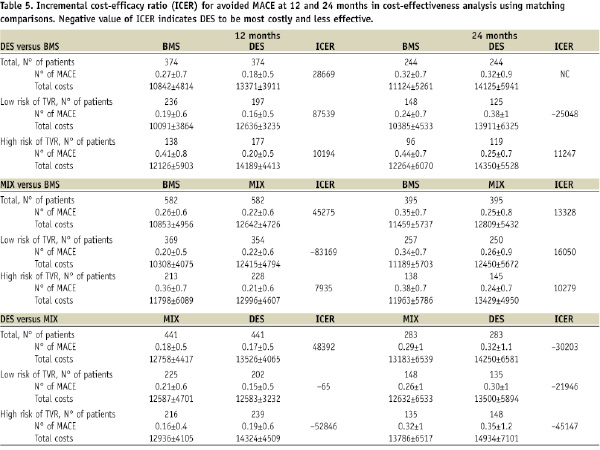
The cost-efficacy ratio of DES or MIX in respect to BMS appeared better in the high risk subgroup, but never becoming dominant (more effective and less expensive); it is noteworthy a possible negative effect in the low risk population in conjunction to higher costs. DES showed a worse cost-effectiveness ratio respect to MIX, especially at two years.
Figures 2, 3, and 4 show the cost-effectiveness results in low and high risk subgroups plotting the bootstrapping analysis on cost-efficacy Cartesian planes and the economic acceptability curves in the three comparisons. At a willingness to pay threshold for any avoided MACE of 20,000 - 30,000 Euro, DES and MIX have a high probability (90-95% at one year and 80-85% at two years) of being cost-effective in respect to BMS only in patients at high risk of TVR, while DES have a very low probability to be cost-effective in respect to MIX (10-15% at one year and 30% at two years) even in the high risk subgroup of patients.
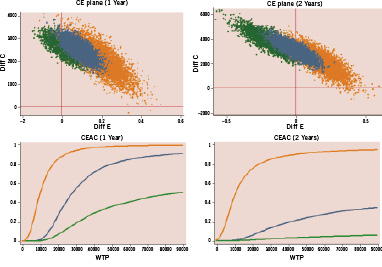
Figure 2. Matched comparison of DES versus BMS: incremental cost-effectiveness (bootstrap analysis) and probability of cost-effectiveness in relation to willingness to pay to prevent one MACE at 1 and 2 years in patients treated with multivessel PCI at low (green) and high (orange) risk of TVR. CE: cost-efficacy; CEAC: cost-efficacy acceptability curve; WTP: willingness to pay.
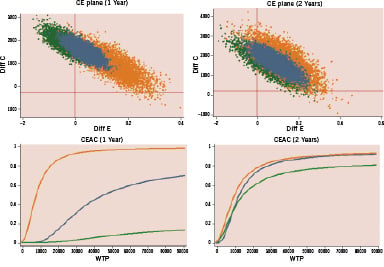
Figure 3. Matched comparison MIX versus BMS: incremental cost-effectiveness (bootstrap analysis) and probability of cost-effectiveness in relation to willingness to pay to prevent one MACE at 1 and 2 years in patients treated with multivessel PCI at low (green) and high (orange) risk of TVR. CE: cost-efficacy; CEAC: cost-efficacy acceptability curve; WTP: willingness to pay.
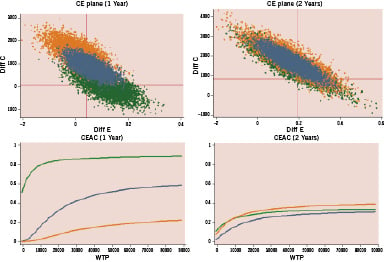
Figure 4. Matched comparison DES versus MIX: incremental cost-effectiveness (bootstrap analysis) and probability of cost-effectiveness in relation to willingness to pay to prevent one MACE at 1 and 2 years in patients treated with multivessel PCI at low (green) and high (orange) risk of TVR. CE: cost-efficacy; CEAC: cost-efficacy acceptability curve; WTP: willingness to pay.
Discussion
The main finding of our study was that in patients undergoing multivessel PCI, DES conferred a clinical advantage over BMS only in patients showing features of high pre-procedural risk of subsequent TVR (Table 3), and exclusive DES employment was not superior to a mixed (DES and BMS in the same patient) strategy. Although the similar rate of TVR in the three groups could suggest that a major reduction of late loss should not be necessary in a wide spectrum of patients, the propensity score adjusted comparisons showed DES and MIX approaches to confer a lower TVR rate in comparison to BMS, exclusively driven by the effect in the high risk subgroups of patients.
The REAL registry experience, already published11 and enlarged in this study, was the first reporting in the literature of a mixed DES/BMS strategy in multivessel PCI. Prolongation of follow-up at two years resulted in the observation of a reduction of DES advantage over BMS between 12 and 24 months. This is particularly evident in the cost-effectiveness analysis that takes into account not only the first adverse event as in the Kaplan Meier curves construction, but all events for each patient. In fact, even in the face of an absolute inferior incidence of events, patients in the DES group experienced more frequently recurrence of multiple events. This may be due to the presence in this group of patients of a more complex anatomical situation (more diseased vessels, more complex lesions) leading to more non-fatal events (AMI and TVR). A reduction of the clinical benefit of DES in respect to BMS after the first year of follow-up was reported also in the ERACI III registry6.
In this registry, BMS and DES costs resulted less than those reported in the literature19-22, with less difference between the two types of stents and a progressive reduction of costs over time. Even with a reduced cost of 12 months follow-up, patients treated with DES consumed more resources than those treated with BMS. This, in conjunction with a higher initial cost, leads to a higher final cost for the groups DES and MIX in comparison to BMS group.
Previous cost-efficacy analyses of the Sirius19 and Taxus20 studies showed an acceptable incremental cost (threshold of standard cost-efficacy for any avoided TVR of 10,000 USD), but observational registry studies with unrestricted DES utilisation21 demonstrated that DES could not result cost efficacious, at least until a buy price of or more than 1,336 Euros. Randomised studies in unselected patients22-24 showed better clinical and cost-efficacy ratio results in high risk patients. Our experience confirms the better economical acceptability of DES when employed in patients at high risk of TVR, with an high probability to avoid one MACE for an expense threshold of 20,000 Euros (Figures 3-5). Moreover, it is of note that in patients at low risk for TVR, there were not only higher costs, but also a possible negative effect on clinical events (Table 5). Lastly, exclusive DES utilisation in respect to a mixed approach did not produce any advantage even in the high risk subgroup, in the presence of increased costs. So our study favours appropriateness of a mixed approach (DES utilisation only in lesions at high risk of restenosis) also from a cost-efficacy perspective.
This study has several limitations. It is observational, not randomised and so the numerous and obvious differences in the clinical, angiographic and procedural characteristics among the three groups have been addressed with the statistical methodology of propensity score adjustment. This may not be sufficient to overcome the basal imbalance, even with good calibration and predictivity values of the model employed.
To correct for the observational nature of the study, cost-effectiveness analysis has been conducted employing statistical matching of homogeneous subgroups that are obviously only a part of the total study population. Group comparison prior to and post matching showed a good balance of patient populations, with few residual differences, notably reduced in magnitude, regarding dyslipidemia, Cx or RCA as treated artery, total lesion length and total stent length (data not shown).
Cost calculation is heavily influenced by local situations, length of stay, and centres’ volume of activity. To minimise these variables we employed the PCI DRG reimbursement as a standard, adding the costs for the type and the number of stents employed. Even with the limitation of the applicability to other realities, our analysis has the advantage of considering DES and BMS costs more updated in respect to those considered in other published studies, and of a concomitant and prolonged clopidogrel therapy.
Economical acceptability curves clearly indicate the probability of DES to become cost-effective in relation to different expenses thresholds. The threshold of 20,000-30,000 Euros for any avoided MACE is arbitrary, but intermediate between that considered for any avoided TVR (in general 10,000 USD) and that for quality-adjusted life year (QALY) (in general 50,000 USD). Being the study retrospective, we lack data about quality of life and calculations of incremental cost for QALY, considered the gold standard of cost-efficacy analysis25.
Conclusions
In this real-world multivessel PCI registry, DES were used in 55% of patients, mainly in patients and in lesions at a higher risk of TVR. The major clinical advantages and a reasonable cost-efficacy of DES were seen only in the subgroup of patients at high risk of TVR. In multivessel PCI, exclusive DES utilisation did not lead to any clinical advantage and had higher costs in respect to a mixed approach at two years.

