
Abstract
Aims: In patients at high risk of bleeding who undergo PCI, the Biolimus A9 polymer-free drug-coated stent (DCS) has superior efficacy and safety compared to a bare metal stent (BMS). We estimated the cost-effectiveness of DCS vs. BMS.
Methods and results: The LEADERS FREE-based economic evaluation estimated service use and quality of life data collected prospectively. The entire trial population was analysed using cost weights from England, France, Germany, Italy, Scotland and Spain. Country-specific QALYs were derived from EQ-5D scores. We estimated cost per event averted and per QALY gained. DCS use resulted in -0.095 cardiac deaths, target vessel MI, stent thrombosis and revascularisation per patient (0.152 vs. 0.237; p<0.001). One-year QALYs were non-significantly higher in the DCS group. Total costs for the index admission were similar among groups. One-year costs using cost weights from each of the six countries, including the additional €300 per DCS stent, ranged from €4,664-8,593 for DCS and €4,845-9,742 for BMS and were lower in the DCS group (England: –428 €, France: –137 €, Germany: –33 €, Italy: –522 €, Scotland: –298 €, Spain: –854 €).
Conclusions: The probability that DCS dominated BMS was >50% in all countries. At a threshold of €10,000 per event averted, DCS had a 98% probability of being cost-effective in all six countries.
Abbreviations
BMS: bare metal stent
CHEERS: Consolidated Health Economic Evaluation Reporting Standards
DAPT: dual antiplatelet therapy
DCS: drug-coated stent
MACE: major adverse cardiac events
PCI: percutaneous coronary intervention
QALY: quality-adjusted life-years
Introduction
The choice of drug-coated stents vs. bare metal stents for patients undergoing percutaneous revascularisation is based upon medical criteria but also has economic implications due to the large number of patients receiving stents worldwide. In populations that are not at increased risk of bleeding, drug-eluting stents were found to be cost-effective compared to bare metal stents in patients at high risk of cardiac events1. At least 15% of patients who undergo percutaneous coronary intervention (PCI) are at increased risk of bleeding2,3, at a cost of US $4,000-14,000 per episode4. Due to their relative or absolute contraindication to prolonged dual antiplatelet therapy (DAPT), these patients at high risk of bleeding are often treated with bare metal stents followed by one month of DAPT only5,6, which is associated with a higher risk of restenosis and reintervention than that observed with the use of a drug-eluting stent7. LEADERS FREE was a double-blind multicentre trial including nearly 2,500 patients at high risk of bleeding comparing BioFreedom™ (Biosensors Europe, Morges, Switzerland), a new Biolimus A9 polymer-free drug-coated stent, to a bare metal stent. The Biolimus A9 drug-coated stent demonstrated superiority at one year in terms of both safety (composite endpoint of death, MI and stent thrombosis of 9.4% vs. 12.9%, p<0.005) and efficacy (clinically indicated target lesion revascularisation of 5.1% vs. 9.8%, p<0.001), all patients being treated with one month only of DAPT followed by single antiplatelet treatment8.
We performed a prospective health economic evaluation alongside the LEADERS FREE trial to understand the cost-effectiveness of these alternative stenting strategies.
Methods
STUDY DESIGN AND ORGANISATION
The design and results of the LEADERS FREE trial have been described previously8. Between December 2012 and May 2014, 2,466 patients at increased bleeding risk underwent randomisation to treatment with a Biolimus A9 (BA9) coated stent or a bare metal stent. The prospective economic evaluation was concurrent with the randomised trial, in accordance with the CHEERS guidelines9. The perspective chosen was that of the healthcare system; resources and quality of life were collected prospectively alongside the clinical trial. Study participants lost to follow-up were included in the analysis. LEADERS FREE was conducted at 68 sites in 20 countries on four continents. The cost weights used in the economic analysis were obtained from countries where the number of patients included allowed precise estimates of country-specific values (England, France, Germany, Italy, Scotland and Spain). We analysed the overall trial population using cost weights from each of the six countries mentioned10-12. LEADERS FREE ClinicalTrials.gov number: NCT01623180.
EFFECTIVENESS
The economic evaluation used components of the safety and efficacy endpoints (cardiac death, target vessel myocardial infarction, target vessel stent thrombosis and target lesion revascularisation) and quality-adjusted life-years (QALY). The use of clinical endpoints as effectiveness criteria is standard in economic evaluations of cardiac interventions1,11, and the use of QALY is recommended by the CHEERS guidelines9. Because the clinical study found homogeneity of safety and efficacy between study locations, we pooled the one-year clinical results from all countries8.
ESTIMATION OF RESOURCE UTILISATION
All costs were assessed from the perspective of the healthcare system. The time horizon was one year. We verified practice heterogeneity between countries to ensure that medical resource utilisation could be pooled for the cost analysis.
Post-procedural days for each initial hospitalisation were recorded prospectively. The follow-up cardiovascular hospitalisations were included in the economic evaluation if at least one concurrent adjudicated cardiac clinical endpoint was recorded between the day before the admission date and the discharge date.
Outpatient resources (doctor visits, medications, non-invasive exams, etc.) were not included in the cost calculations as all patients were prescribed identical scheduled follow-up in both arms and we assumed that all significant events would result in a hospital admission. For the same reason, hospitalisations for bleeding events were not counted either, as the antiplatelet therapy regimen was identical in the two groups, and bleeding events were not different between the two groups.
ESTIMATION OF UNIT COSTS
Total hospital costs were calculated as daily hospital costs multiplied by the length of stay. Either PCI or cardiac-related daily costs were applied to re-hospitalisations depending on whether a PCI procedure was performed. Costs were reported with the hospital costs of each country. Costs were reported in euros; British pounds were converted to euros using a purchasing power parity of 1.34. As stent prices vary per country, we calculated an average price mark-up of €300 for a drug-coated stent over a bare metal stent. Similar stent prices were used in all countries, as in the SYNTAX trial13.
QUALITY OF LIFE
The main outcome was expressed as QALY assessed directly from patients at baseline and at one year with the EuroQOL EQ-5D-5L questionnaire14 health status instrument, and converted to country-specific utility weights for each studied country (UK weights were selected for both England and Scotland). The initial weight reflecting the quality of life at the time of the PCI was attributed to the entire month following the initial procedure. The trial did not record the EuroQOL at regular intervals during the follow-up period. We assumed that each adverse event would be associated with a utility decrement and therefore attributed the initial weight to the month following each adverse event, while the final utility weight at one year was attributed to the remaining time, except when the final utility weight was worse than at inclusion: in that case the initial weight was not used to decrement utility. Missing follow-up utilities because of premature death were imputed through linear regression prediction to the time of death. QALY were calculated for each patient as the time-weighted average of utility values.
COST-EFFECTIVENESS
A cost-effectiveness analysis was conducted to estimate incremental costs per incremental QALY for the primary analysis and clinical endpoints for the secondary analysis15. Costs and QALY were not discounted due to the short time horizon.
UNCERTAINTY ANALYSIS
Bootstrapping (10,000 replicates) was used to estimate uncertainty in the joint distribution of costs, clinical endpoints and QALY for each treatment group. Results were presented on the cost-effectiveness plane and as a cost-effectiveness acceptability curve16.
STATISTICAL ANALYSIS
Categorical data are reported as frequencies, and continuous data are reported as mean±SD. Discrete variables were compared by use of Fisher’s exact tests. Continuous variables (including costs) were compared using bootstrap hypothesis testing in order to avoid relying on normality assumptions17. Count data were compared with a Poisson model. All analyses were performed with R version 3.2.318, and results were plotted with the ggplot2 R package19.
Results
PATIENT POPULATION
A total of 2,466 patients underwent randomisation (1,239 were assigned to the drug-coated stent and 1,227 were assigned to the bare metal stent) from December 2012 to May 2014. Of the 2,432 patients who underwent PCI, 2,385 (98.1%) were followed until death or 390 days.
EFFECTIVENESS
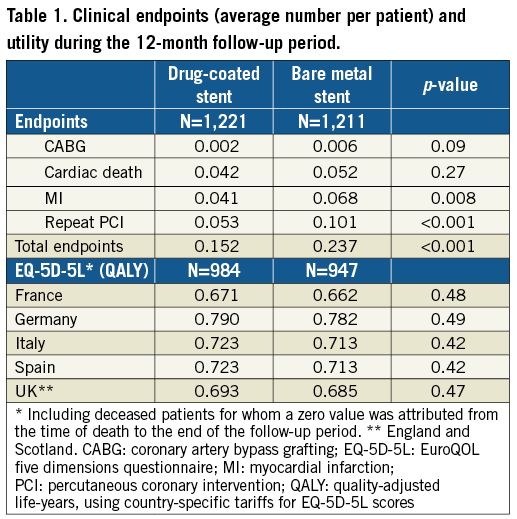
In accordance with the clinical results8, the number of clinical safety and efficacy endpoints per patient was lower in the drug-coated stent group with an average reduction of 0.095 events per patient (0.152 vs. 0.237; p<0.001) (Table 1).
UTILITY WEIGHTS AND QALY
QALY of the one-year follow-up were non-significantly higher in the drug-coated stent group, ranging from 0.671 to 0.790 across countries vs. 0.662 to 0.713 for the bare metal stent group (difference from 0.008 to 0.010). Utilities and cumulative QALY are presented in Table 1.
TREATMENT COSTS
Resource use for the initial revascularisation procedures is summarised in Table 2. The average number of stents, angioplasty balloons and guiding wires was identical in both groups. Total procedural hospitalisation costs were similar between groups (non-significant cost differences in favour of the drug-coated stent group: England –160 €, France –202 €, Germany –148 €, Italy –226 €, Scotland –139 €, Spain –295 €) (Table 3). The total stent cost was higher for the drug-coated group based on an average of 1.8 stents per patient (cost difference: €557) (Table 3).
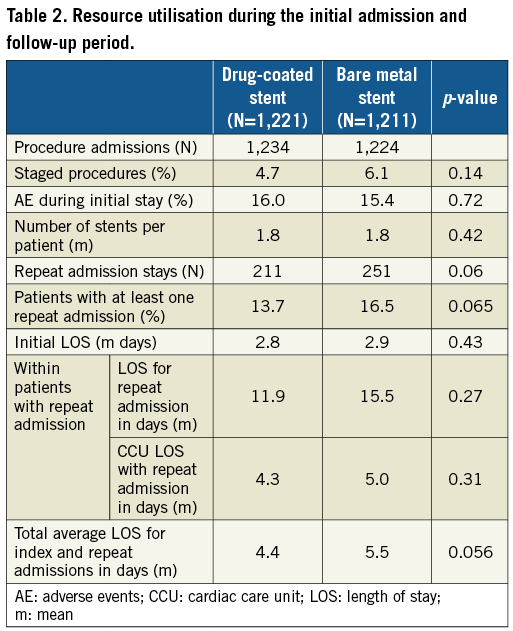
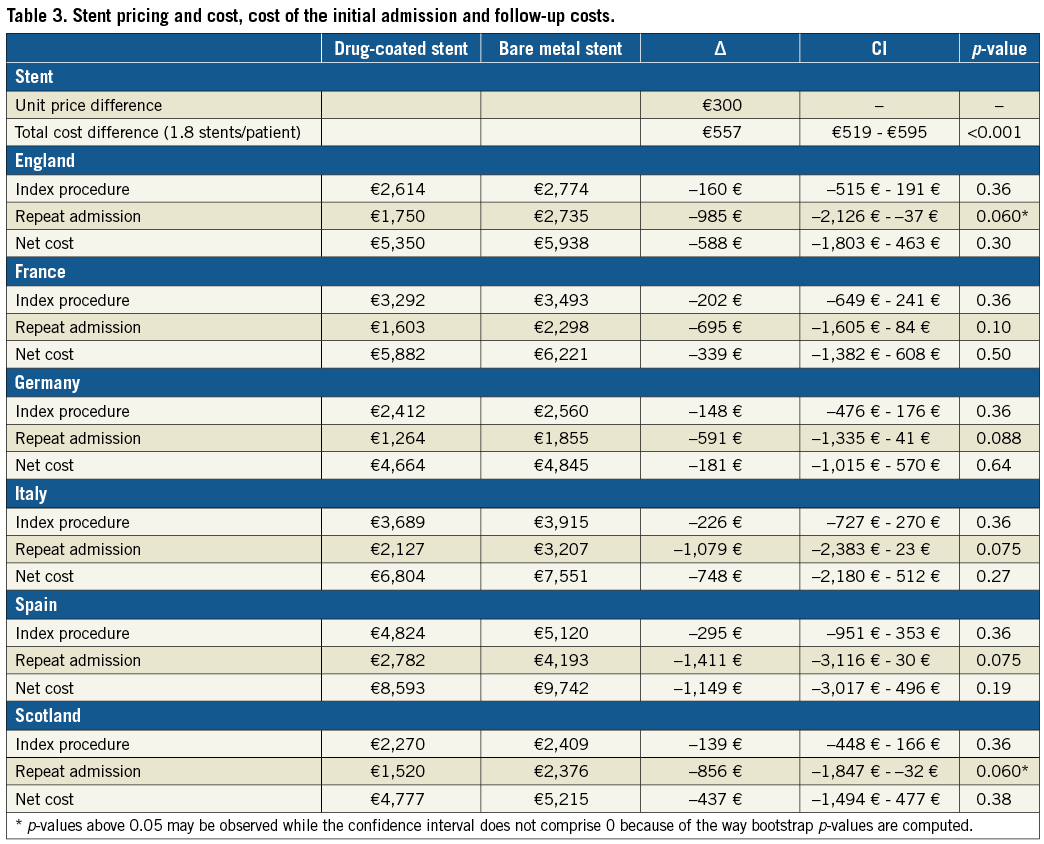
The difference in rates of cardiac death, target vessel myocardial infarction, target vessel stent thrombosis, target lesion revascularisation and length of stay was consistently in favour of the drug-coated stent group (Table 2), resulting in higher post-procedural hospital costs in the bare metal stent group in all countries (cost differences in favour of the drug-coated stent group: England –985 €, France –695 €, Germany –591 €, Italy –1,079 €, Scotland –856 €, Spain –1,411 €) (Table 3). The total one-year costs, including stent costs, were lower in the drug-coated stent group (cost differences: England –588 €, France –339 €, Germany –181 €, Italy –748 €, Scotland –437 €, Spain –1,149 €) (Table 3). None of these differences reached statistical significance.
COST-EFFECTIVENESS AND SENSITIVITY ANALYSES
The probability that drug-coated stents dominated bare metal stents (i.e., were both more effective and less costly) depended on the country studied and on the outcome chosen (QALY or clinical endpoints), but remained above 50% in all situations. In the QALY analysis, drug-coated stent dominance was found in: England 65%, France 57%, Germany 52%, Italy 69%, Scotland 62%, and Spain 72%. In the clinical events analysis, drug-coated stent dominance was found in: England 85%, France 75%, Germany 67%, Italy 87%, Scotland 80%, and Spain 91% (Figure 1).
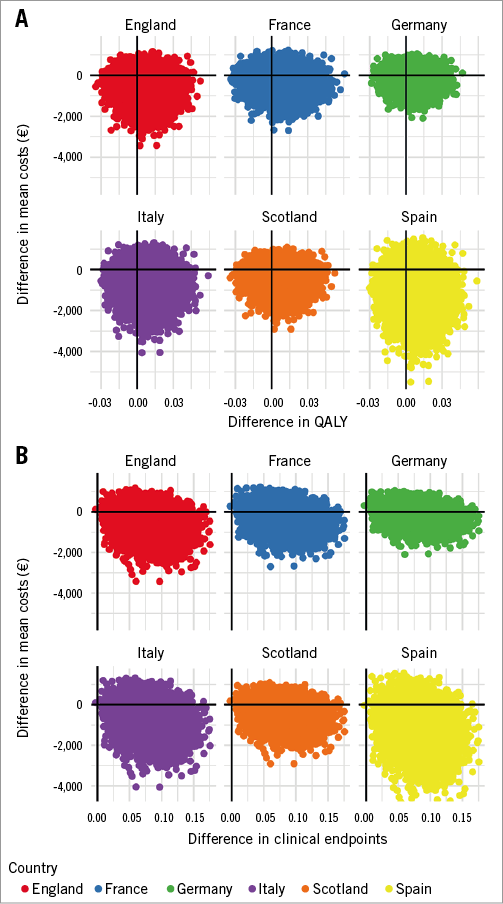
Figure 1. Distribution of the incremental cost and effectiveness on the cost-effectiveness plane. Distribution obtained by bootstrapping on the cost-effectiveness plane for QALY (A) and clinical endpoints (B) representing the incremental effectiveness of drug-coated stents over bare metal stents on the X-axis, and the incremental cost on the Y-axis.
If stakeholders were ready to pay €50,000 per QALY, the probability of being cost-effective was 70-75% for all countries. At a threshold of €10,000 per clinical event averted, drug-coated stents had a 98% probability of being cost-effective in all countries (Figure 2).
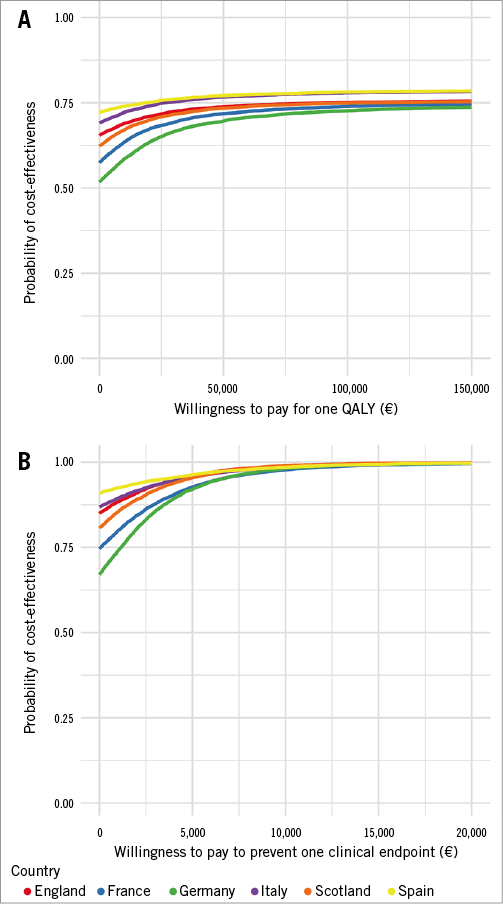
Figure 2. Cost-effectiveness acceptability curve: probability of cost-effectiveness in relation to willingness to pay. Probability of drug-coated stents being more cost-effective than bare metal stents on the Y-axis, depending on the stakeholder’s willingness to pay for QALY (A) or an improvement in clinical endpoints (B) on the X-axis.
We found that the initial admission length of stay was slightly higher for the bare metal stent group, resulting in higher hospitalisation costs for the index procedure. Since the investigators were blinded, there was no obvious clinical explanation for this non-significant difference except random fluctuation. To ensure strict comparison of effects and costs, we set the difference in post-procedural costs at €0 and found that the results of the economic evaluation remained similar (England: –428 €, France: –137 €, Germany: –33 €, Italy: –522 €, Scotland: –298 €, Spain: –854 €).
Discussion
LEADERS FREE was the first study to compare directly the clinical and economic outcomes of drug-coated and bare metal stents in patients at high risk of bleeding. Use of the drug-coated stent was associated with a better safety and reduced events rate at one year. This economic substudy provides information on the efficient stenting strategy in such patients. The average total one-year cost of drug-coated stent patients was consistently lower than the cost of bare metal stent patients, making the drug-coated intervention cost-saving. Overall, using cost weights from each of the six countries studied, we found that the drug-coated stent dominated the bare metal stent despite higher initial stent costs.
This dominance of drug-coated stents was explained by the better results in safety and efficacy: hospital costs were reduced in all countries following fewer and shorter re-admissions, and lower costs. Between-country variations were mostly explained by differences in hospital costs; higher hospital costs were associated with a higher probability of drug-coated stents being dominant. Results were less striking for QALY than for clinical endpoint prevention due to the low sensitivity of the EQ-5D-5L to detect quality-of-life improvements in coronary patients over a one-year period. These results are in line with the NORSTENT study (with a five-year follow-up) that did not find significant differences in QALY while the rate of repeat revascularisation differed between groups20.
The LEADERS FREE clinical trial found the drug-coated stent to be significantly superior in terms of safety (composite endpoint of death, MI and stent thrombosis of 9.4% vs. 12.9%, p<0.005) and efficacy (clinically indicated target lesion revascularisation of 5.1% vs. 9.8%, p<0.001), while the difference in hospital admissions for clinical events during that same period did not reach statistical significance. This is explained by the fact that several adverse events resulted in only one admission (e.g., stent thrombosis and PCI) that reduced the 10% difference in total clinical endpoints to a 3% difference in total re-admissions (Table 1, Table 2). Total costs were mainly driven by the costs of the initial procedure (hospital stay and stent cost); the cost of repeat admissions represented on average ¼ to ⅓ of total one-year costs. Thus, the difference in one-year costs was not proportional to the difference in clinical effects.
Cost-effectiveness ratios reported in the BASKET trial were €64,732 to prevent one major adverse cardiac event, and €40,467 per QALY gained, higher than our results where drug-coated stents were more effective and less costly. However, in the subgroup of high-risk patients, the efficiency was very much improved. Our findings are consistent with other studies that showed cost-effectiveness ratios driven by stent prices and their number1,21,22. LEADERS FREE reported about the same number of stents per lesion as the BASKET trial1, while other trials had fewer stents per patient23,24.
Economic evaluations of drug-eluting stents versus bare metal stents consistently showed that the former are more effective in reducing major adverse cardiac events (MACE) and also more costly. In the SIRIUS trial, the base-case analysis found an incremental cost-effectiveness ratio for sirolimus-eluting stenting at US $1,650 per repeat revascularisation avoided, a cost utility of US $27,540 per QALY gained,
Our decision to pool results for efficacy and resource use but to separate resource costs per country is in line with findings that the factor most frequently cited as generating variability in economic results between locations was the unit costs associated with particular resources26.
Limitations
Repeat admissions were selected to be included in the cost analysis when they occurred at the same date as a cardiac event described as safety or efficacy endpoints in the trial data. We did not use the hospitals’ discharge data principal diagnoses because: 1) the coding rules of principal diagnoses differ between countries, and 2) principal diagnoses are sometimes coded according to economic rather than clinical criteria. We used the same mark-up in stent price for all countries because the exact per-country prices were not disclosed by the manufacturers for confidentiality reasons.
Conclusions
Taking into account the country level differences in hospital costs, we found that using Biolimus A9 drug-coated stents in patients at high bleeding risk was cost-effective compared to bare metal stents.
| Impact on daily practice Our study showed that, in patients at high bleeding risk, DCS were probably (>50%) cost-saving in six different European countries, and were almost certainly (>98%) cost-effective with a willingness to pay at €10,000 per clinical event avoided. These results are needed for the DCS price discussion in European countries. |
Acknowledgements
We thank Professor Hervé Maisonneuve for his medical writing recommendations for this article.
Funding
The LEADERS FREE trial and economic evaluation were supported by Biosensors Europe.
Conflict of interest statement
I. Durand-Zaleski reports receiving personal fees from Medtronic and Boston Scientific during the conduct of the study. She is an invited speaker and undertakes educational activities at EuroPCR. K. Oldroyd reports personal fees from Biosensors, outside the submitted work. P. Urban and S. Greene are paid consultants for Biosensors. U. Windhovel is an employee of CERC and M-C. Morice is the CEO of CERC. The other authors have no conflicts of interest to declare.

