
Introduction
In patients with acute coronary syndrome (ACS) undergoing PCI, bleeding is associated with increased mortality1. An abbreviated dual antiplatelet therapy (DAPT) is recommended after drug-eluting or bare metal stent (BMS) implantation for patients at high bleeding risk (HBR)2. The LEADERS FREE trial3 demonstrated superiority for safety and efficacy endpoints of a polymer-free Biolimus A9™ (BA9) drug-coated stent (DCS) over a similar BMS for patients at HBR treated with one-month DAPT. The one-year outcome of a pre-specified substudy of patients presenting with ACS has been reported4.
The aim of this analysis was to evaluate whether the demonstrated superiority in safety and efficacy of the DCS compared to BMS at one year was sustained up to 24 months in HBR patients with ACS.
Methods
PATIENTS AND METHODS
The study includes all patients presenting with ACS at HBR from the modified intention-to-treat analysis of the LEADERS FREE trial3,4, a randomised, double blind trial. Patients undergoing PCI were assigned to a polymer-free BA9 DCS (BioFreedom™; Biosensors Europe, Morges, Switzerland) or a similar BMS (Gazelle™; Biosensors Interventional Technologies, Singapore, Singapore). All patients were assigned to one month of DAPT only, followed by long-term single antiplatelet therapy (SAPT).
STUDY ENDPOINTS
The primary safety endpoint consisted of cardiac death, myocardial infarction (MI), and definite or probable stent thrombosis (ST). The primary efficacy endpoint was defined as clinically driven target lesion revascularisation (TLR).
Results
PATIENTS AND PROCEDURES
Six hundred and fifty-nine patients5 presenting with ACS (554 NSTEMI and 105 STEMI) were followed for two years after assignment to DCS (330 patients) or BMS (329 patients) treatment.
PRIMARY ENDPOINTS
At two years, the primary safety endpoint had occurred more frequently in the BMS than in the DCS group (Figure 1). This difference was driven by events occurring within the first year. Between year one and year two, the primary safety endpoint occurred in 19 patients (DCS vs. BMS: 8 vs. 11 patients, p=0.560).
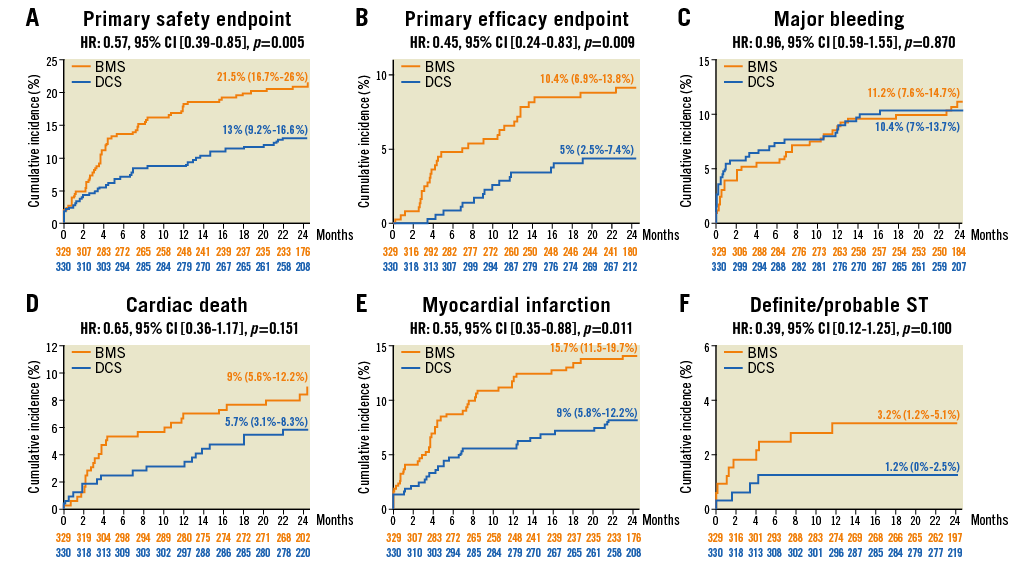
Figure 1. Time-to-event analysis at two years. A) Primary safety endpoint. B) Primary efficacy endpoint. C) Major bleeding. D) Cardiac death. E) Myocardial infarction. F) Definite/probable ST.
The primary efficacy endpoint was reached by 10.4% in the BMS and 5.0% in the DCS group (Figure 1). This difference was driven by events occurring within the first year. Between year one and year two, the primary efficacy endpoint occurred in seven patients (DCS vs. BMS: 3 vs. 4 patients, p=0.152) (Figure 2).
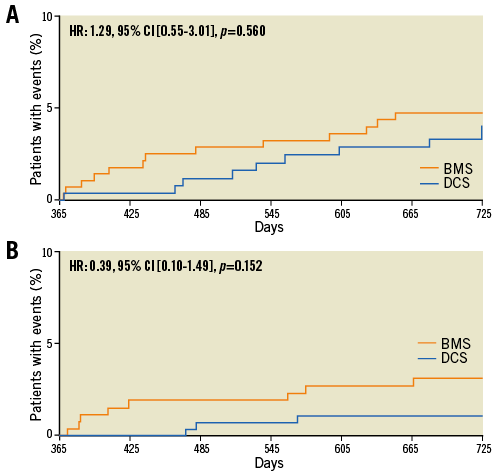
Figure 2. Landmark analysis for the primary safety and primary efficacy endpoints. The Kaplan Meier time-to-event curves show the cumulative percentage of patients who reached the primary safety endpoint (A) and the primary efficacy endpoint (B) for the first time between day 365 and day 730 during follow-up.
BLEEDING
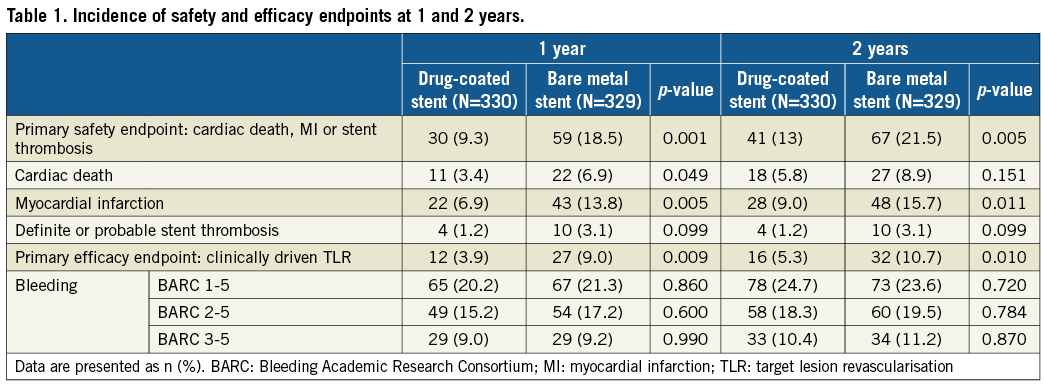
The Bleeding Academic Research Consortium (BARC) 3-5 bleeding rates were high and similar between groups (Table 1).
Details of antiplatelet and oral anticoagulation regimen adherence during follow-up are provided in the Supplementary Appendix, and in Supplementary Table 1.
Discussion
In this analysis of the LEADERS FREE ACS substudy of patients at HBR and with ACS who received only one month of DAPT, the superiority of the BA9 DCS over a BMS was preserved up to two years for both the primary efficacy and the primary safety endpoints. DCS treatment showed a lower rate of MI and a trend towards a lower ST rate, whereas cardiac death was not statistically different between treatment groups.
Short duration DAPT used to be considered a major advantage of using BMS. There are compelling data; however, this comes at the cost of reduced safety and efficacy. This study demonstrated safety and efficacy superiority of DCS compared to BMS at one year and two years. This was also true in patients receiving triple therapy compared to patients on DAPT.
Bleeding rates were high and the highest bleeding rates were observed during the first month while patients were on DAPT. This underlines the need for shortening the duration of DAPT. A prolonged DAPT regimen might have led to an even higher bleeding rate and a detrimental outcome. In fact, previous studies have suggested increasing mortality with prolonged DAPT especially in HBR patients5.
Limitations
This predefined LEADERS FREE ACS substudy did not use a sub-randomisation and is insufficiently powered for clinical endpoints.
– Given the unique properties of the studied DCS, our observations should not be extrapolated to other drug-eluting stents or DCS, or to stents with different drug-elution kinetics.
– The trial was designed to compare two strategies using a guideline-recommended minimal one-month DAPT course for BMS implantation and therefore no conclusions can be made concerning the optimal duration of DAPT for HBR patients after DCS implantation.
Conclusion
In HBR patients presenting with ACS undergoing PCI followed by one-month DAPT, the safety and efficacy benefits of a BA9 DCS compared to BMS were sustained up to 24 months. This study provides further evidence discouraging the use of BMS in HBR patients with ACS.
| Impact on daily practice This trial of HBR patients presenting with ACS undergoing PCI provides evidence that the use of BMS in ACS, in our view, can no longer be recommended. The BA9 DCS followed by one-month DAPT provides a safe and efficient treatment of ACS patients with HBR. |
Funding
This work was funded by Biosensors Europe.
Conflict of interest statement
C. Naber reports receiving personal fees from Abbott, Biosensors, Biotronik, Medtronic, Elixir, REVA and MicroPort, and is a shareholder of CERC, the contract research organisation (CRO) responsible for running the LEADERS FREE study. P. Urban reports receiving personal fees from Biosensors Europe, Edwards Lifesciences, Terumo, Abbott Vascular, Quest Medical, and is medical co-director and a shareholder of CERC. A. Abizaid reports receiving grants from Abbott, Medtronic, Elixir, and Riva. S. Pocock reports receiving personal fees from Biosensors Europe SA. C. Dubois reports receiving personal fees from Boston Scientific, Edwards Lifesciences, and Medtronic, and grants from Abbott Vascular and Biotronik. S. Copt and H.P. Stoll are employees of Biosensors International. M.C. Morice is the CEO of and a shareholder of CERC. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Methods, Results and Discussion.
Supplementary Table 1. Antithrombotic therapy during follow-up.
To read the full content of this article, please download the PDF.

