Abstract
Background: The impact of 1-month dual antiplatelet therapy (DAPT) followed by aspirin monotherapy according to clinical presentation has not been elucidated.
Aims: This study aimed to compare the impact of 1-month DAPT followed by aspirin monotherapy after polymer-free drug-coated stent (PF-DCS) implantation (1-month DAPT after PF-DCS) vs 6-12-month DAPT followed by aspirin monotherapy after biodegradable polymer drug-eluting stent (BP-DES) implantation (6-12-month DAPT after BP-DES) according to clinical presentation.
Methods: This is a post hoc analysis of the One-Month DAPT trial. The primary outcome was the composite of major adverse cardiac and cerebrovascular events (MACCE; a composite of cardiac death, non-fatal myocardial infarction, target vessel revascularisation, and stroke) and major bleeding.
Results: Among 1,828 patients with stable coronary artery disease (CAD), 1-month DAPT after PF-DCS resulted in lower rates of the primary outcome than 6-12-month DAPT after BP-DES (3.9% vs 6.5%; hazard ratio [HR] 0.59, 95% confidence interval [CI]: 0.39–0.90; p=0.012). However, among 1,192 patients with acute coronary syndrome (ACS), the rates of the primary outcome were not significantly different between the two therapy groups (5.6% vs 3.6%; HR 1.57, 95% CI: 0.91–2.70; p=0.102) and a significant interaction was observed between therapy and clinical presentation regarding the primary outcome (Pint=0.005). A significant interaction was observed in MACCE (Pint=0.016), but not in major bleeding (Pint=0.276).
Conclusions: In patients undergoing drug-eluting stent implantation for non-complex lesions, the benefits of 1-month DAPT followed by aspirin monotherapy for a composite of ischaemic and bleeding outcomes were found in patients with stable CAD, but not in those with ACS. ClinicalTrials.gov: NCT02513810.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor has been recommended after drug-eluting stent (DES) implantation to prevent atherothrombotic events; however, issues have been raised regarding increased bleeding risk, medical costs, and non-compliance related to prolonged DAPT123. Therefore, previous trials have examined experimental antiplatelet therapy strategies of short-term DAPT followed by single antiplatelet therapy to balance the ischaemic and bleeding risks4567891011. Although different strategies for DAPT after DES implantation have been recommended depending on clinical presentation, with a relatively shorter duration of DAPT in stable coronary artery disease (CAD) compared to acute coronary syndrome (ACS), studies regarding the impact of short-term DAPT followed by single antiplatelet therapy in patients with stable CAD are limited12312. Recently, the one-month dual antiplatelet therapy followed by aspirin monotherapy after polymer-free drug-coated stent implantation (One-Month DAPT) trial demonstrated the non-inferiority of 1-month DAPT followed by aspirin monotherapy after polymer-free drug-coated stent (PF-DCS) implantation, compared to 6-12-month DAPT after biodegradable polymer drug-eluting stent (BP-DES) implantation for the 1-year composite of ischaemic and bleeding outcomes in patients undergoing percutaneous coronary intervention (PCI) for non-complex lesions13. Thus, the aim of the present post hoc analysis of the One-Month DAPT trial was to evaluate the impact of 1-month DAPT followed by aspirin monotherapy after PF-DCS implantation compared to 6-12-month DAPT after BP-DES implantation, according to clinical presentation.
Methods
Study population and design
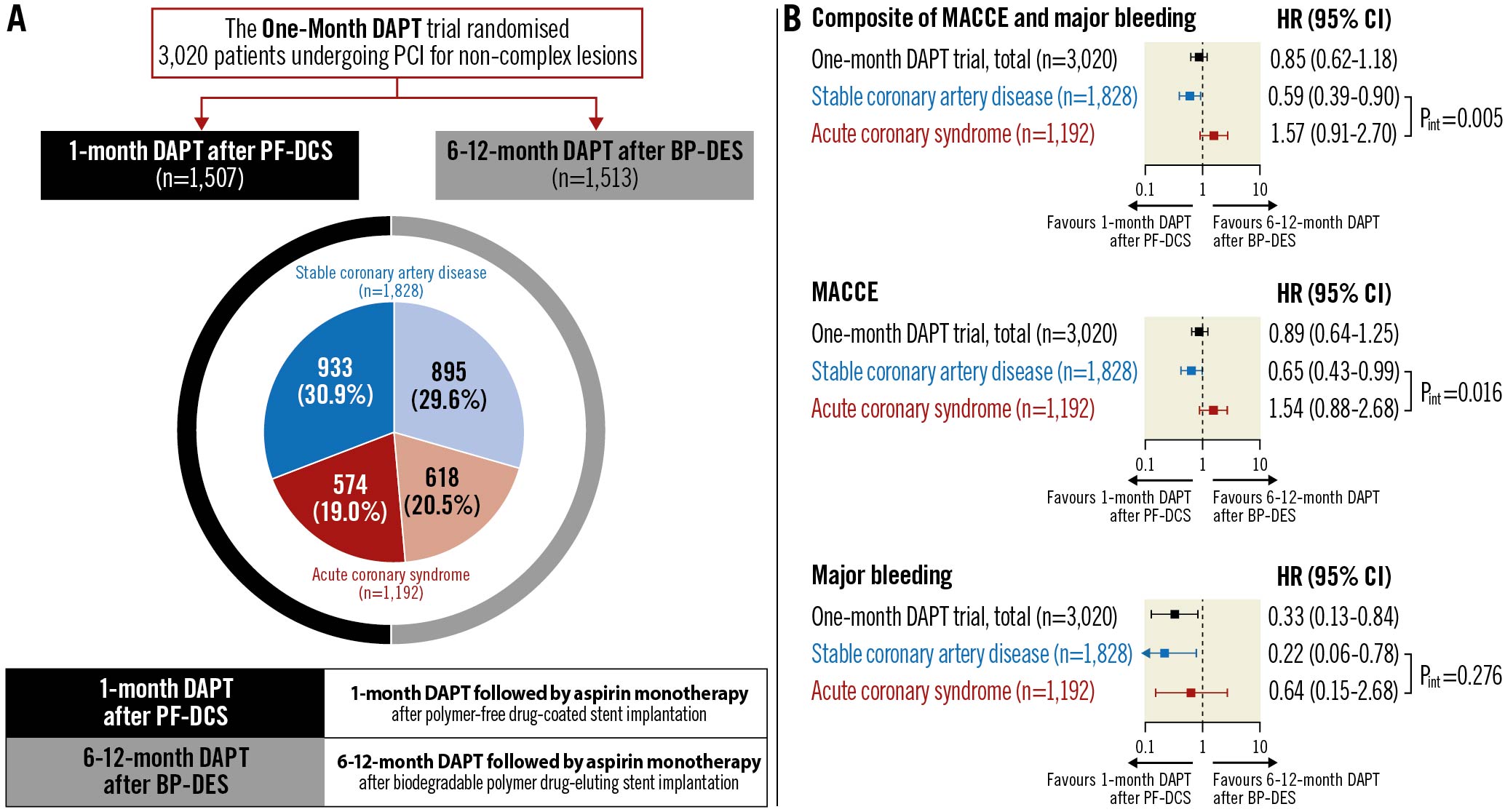
The study design and rationale for the One-Month DAPT trial have been previously described in detail13. Briefly, the multicentre, prospective, open-label, randomised, non-inferiority trial evaluated 1-month DAPT followed by aspirin monotherapy after PF-DCS implantation (1-month DAPT after PF-DCS) vs 6-12-month DAPT followed by aspirin monotherapy after BP-DES implantation (6-12-month DAPT after BP-DES) in 3,020 patients at 23 centres in the Republic of Korea13. Patients who underwent non-emergent PCI for de novo coronary lesions were eligible for participation in the trial and those with complex lesion morphologies, including aorto-ostial, unprotected left main, chronic total occlusion, graft, thrombosis, heavily calcified, or extremely tortuous lesions, were excluded13. The DAPT after the index PCI consisted of aspirin (100 mg) and clopidogrel (75 mg) once per day13. The PF-DCS used in the study was a polymer-free biolimus A9-coated stent (BioFreedom; Biosensors International)13. The BP-DES used in the study was either a stainless-steel biolimus A9-eluting stent with a biodegradable polymer (BioMatrix NeoFlex; Biosensors International) or a cobalt-chromium sirolimus-eluting stent (Ultimaster; Terumo), based on the discretion of the operator13. Consenting patients were randomised in a 1:1 ratio to either of the therapies and observed up to 12 months. In this analysis, patients in the One-Month DAPT trial were classified into two groups according to the clinical presentation at index PCI as follows: those with stable CAD and those with ACS (Figure 1)1214. The trial was approved by the institutional review board of each participating centre and followed the ethical principles of the Declaration of Helsinki. All patients provided written informed consent before participation in the trial.
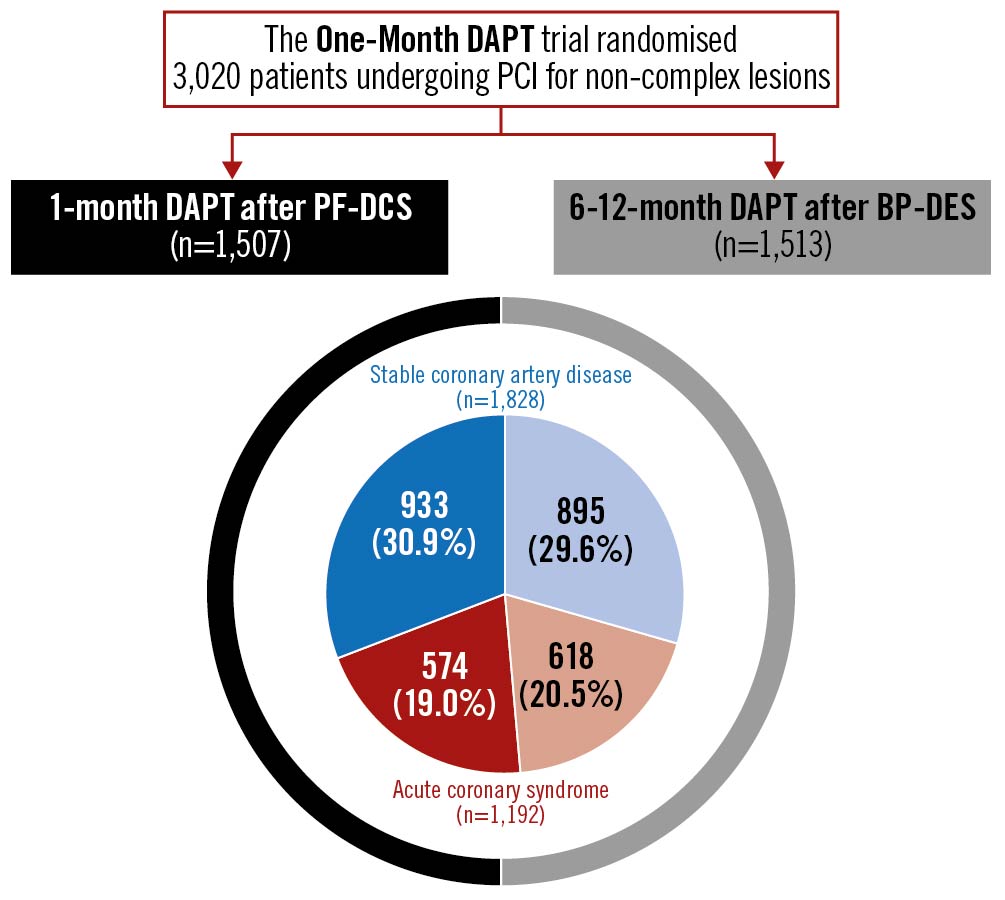
Figure 1. Distribution of One-Month DAPT trial patients by clinical presentation and therapy strategy. BP-DES: biodegradable polymer drug-eluting stent; DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention; PF-DCS: polymer-free drug-coated stent.
Study outcomes and definitions
The primary outcome was the composite of major adverse cardiac and cerebrovascular events (MACCE) and major bleeding at 12 months after the index PCI. MACCE included the composite of cardiac death, non-fatal myocardial infarction, target vessel revascularisation, and stroke. The key secondary outcomes were MACCE and major bleeding.
Clinical events were defined according to the Academic Research Consortium1315. All deaths were considered as cardiac deaths unless a definite non-cardiac cause could be established. Myocardial infarction after discharge from hospital was defined as the presence of clinical symptoms, electrocardiographic changes, or abnormal findings on imaging studies, combined with an increase in creatine kinase-MB fraction above the upper normal limits or an increase in troponin T or I to greater than the 99th percentile of the upper normal limit161718. Ischaemia-driven target vessel revascularisation was defined as repeat PCI or bypass surgery of any segment within the coronary vessel treated during the index PCI, with either of the following: (1) ischaemia symptoms or a positive stress test and angiographic diameter stenosis >50% by quantitative coronary angiographic analysis; or (2) angiographic diameter stenosis >70% by quantitative coronary angiographic analysis, with or without ischaemia symptoms or a positive stress test17. All cerebrovascular events including transient ischaemic attacks and reversible ischaemic neurologic deficits were considered as stroke and further classified as an ischaemic or a haemorrhagic stroke. Definite or probable stent thrombosis was defined according to the recommendations of the Academic Research Consortium16. In this post hoc analysis, major bleeding was defined according to the Thrombolysis in Myocardial Infarction (TIMI) criteria as follows: intracranial bleeding, haemorrhage with a haemoglobin decrease of at least 5 g/dL, or fatal bleeding that resulted in death within 7 days19. Routine follow-up of coronary angiography was not recommended in the trial. Adverse events, including ischaemic and bleeding events, were categorised by an independent clinical event committee blinded to the therapy assignments and primary results of the trial.
Statistical analyses
Primary analyses were performed based on the intention-to-treat population. Continuous variables were reported as mean±standard deviation or median (interquartile range) and compared using Student's t-test or the Mann-Whitney U test. Categorical variables were reported as numbers (percentages) and compared using χ2 tests or Fisher’s exact test. Event rates regarding study outcomes at 12 months were estimated using Kaplan-Meier survival analysis and compared using log-rank tests. Since patients may experience more than one component of the adverse events, each patient was assessed until the occurrence of the first event and only once during the analysis. Hazard ratios (HRs) with 95% confidence intervals (CIs) were computed using Cox regression analysis. To assess whether therapy effects (1-month DAPT after PF-DCS vs 6-12-month DAPT after BP-DES) differ according to clinical presentation (stable CAD vs ACS), formal interaction tests between the therapy and clinical presentation on the clinical outcomes were performed using Cox regression analysis. Prespecified 1-month landmark analyses were performed in patients who were alive at 31 days and did not encounter adverse events of the specific type nor were censored before this landmark13. Moreover, exploratory analyses after excluding the patients with acute myocardial infarction at index PCI were performed13. All tests were two-sided, and p<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS, version 25.0 (IBM) and R 3.5.3 software (R Foundation for Statistical Computing).
Results
Baseline characteristics
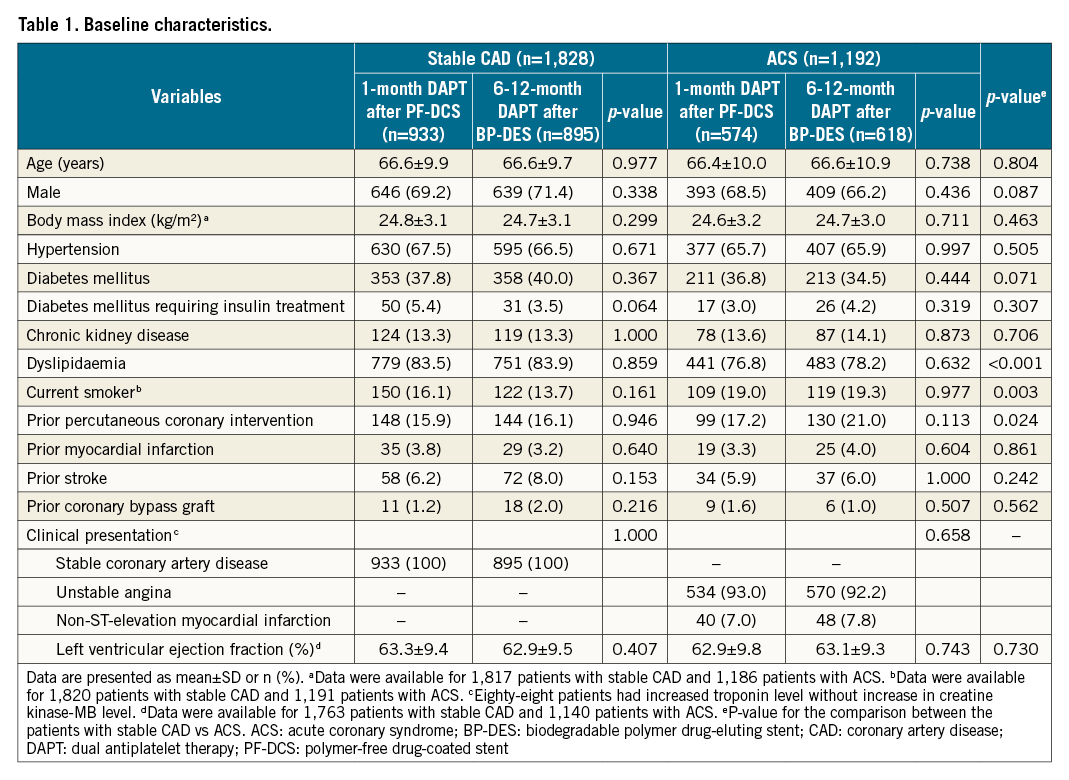
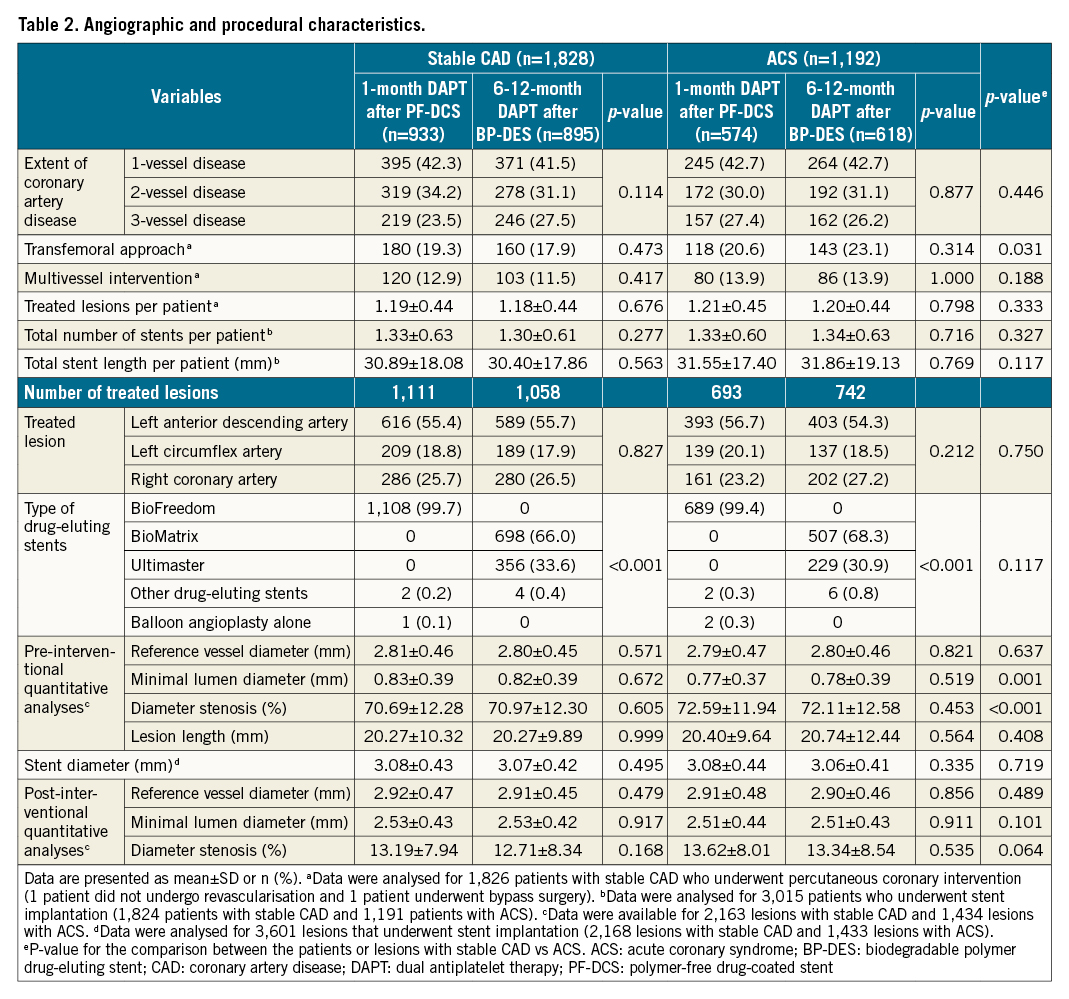
Between December 2015 and September 2019, 3,020 patients were enrolled in the One-Month DAPT trial: 1,828 patients presented with stable CAD, and 1,192 patients presented with ACS. The baseline clinical, angiographic, and procedural characteristics according to the clinical presentation are described in Table 1 and Table 2. The stable CAD group had a lower proportion of current smokers, patients who had undergone prior PCI, patients in whom the transfemoral approach had been used, and patients with smaller diameter stenosis on pre-interventional quantitative analyses than the ACS group. In contrast, patients with stable CAD had a higher proportion of dyslipidaemia and larger minimal luminal diameter on pre-interventional quantitative analyses. Among the patients with stable CAD, 933 patients were assigned to the 1-month DAPT after PF-DCS group, and 895 patients were assigned to the 6-12-month DAPT after BP-DES group. Among the patients with ACS, 574 patients were assigned to the 1-month DAPT after PF-DCS group, and 618 patients were assigned to the 6-12-month DAPT after BP-DES group. Baseline characteristics did not differ between the two therapy groups in patients with stable CAD and ACS (Table 1, Table 2).
Clinical outcomes by clinical presentation and therapy strategy
The effects of 1-month DAPT after PF-DCS compared to 6-12-month DAPT after BP-DES in patients with stable CAD and ACS are presented in Table 3 and Figure 2. With regard to the primary outcome, in patients with stable CAD, 1-month DAPT after PF-DCS resulted in lower rates of the primary outcome compared to 6-12-month DAPT after BP-DES (3.9% vs 6.5%; HR 0.59, 95% CI: 0.39-0.90; p=0.012) (Figure 2A). However, in patients with ACS, the rates of the primary outcome were not significantly different between 1-month DAPT after PF-DCS and 6-12-month DAPT after BP-DES (5.6% vs 3.6%; HR 1.57, 95% CI: 0.91-2.70; p=0.102) (Figure 2B). A significant interaction was observed between therapy and clinical presentation, indicating a different therapy effect of 1-month DAPT after PF-DCS according to clinical presentation (Pint=0.005) (Figure 2C). Regarding secondary outcomes, in patients with stable CAD, 1-month DAPT after PF-DCS resulted in lower rates of MACCE compared to 6-12-month DAPT after BP-DES (3.9% vs 6.0%; HR 0.65, 95% CI: 0.43-0.99; p=0.044). However, in patients with ACS, rates of MACCE were not significantly different between the two therapy groups (5.3% vs 3.4%; HR 1.54, 95% CI: 0.88-2.68; p=0.128) and a significant interaction was observed, which indicates a different therapeutic effect of 1-month DAPT after PF-DCS according to clinical presentation (Pint=0.016). In addition, in patients with stable CAD, 1-month DAPT after PF-DCS resulted in lower rates of major bleeding compared to 6-12-month DAPT after BP-DES (0.3% vs 1.5%; HR 0.22, 95% CI: 0.06-0.78; p=0.010). However, in patients with ACS, rates of major bleeding did not significantly differ between the two therapy groups (0.5% vs 0.8%; HR 0.64, 95% CI: 0.15-2.68; p=0.537), but no significant interaction was observed, which indicates a similar therapeutic effect of 1-month DAPT after PF-DCS, irrespective of clinical presentation (Pint=0.276).
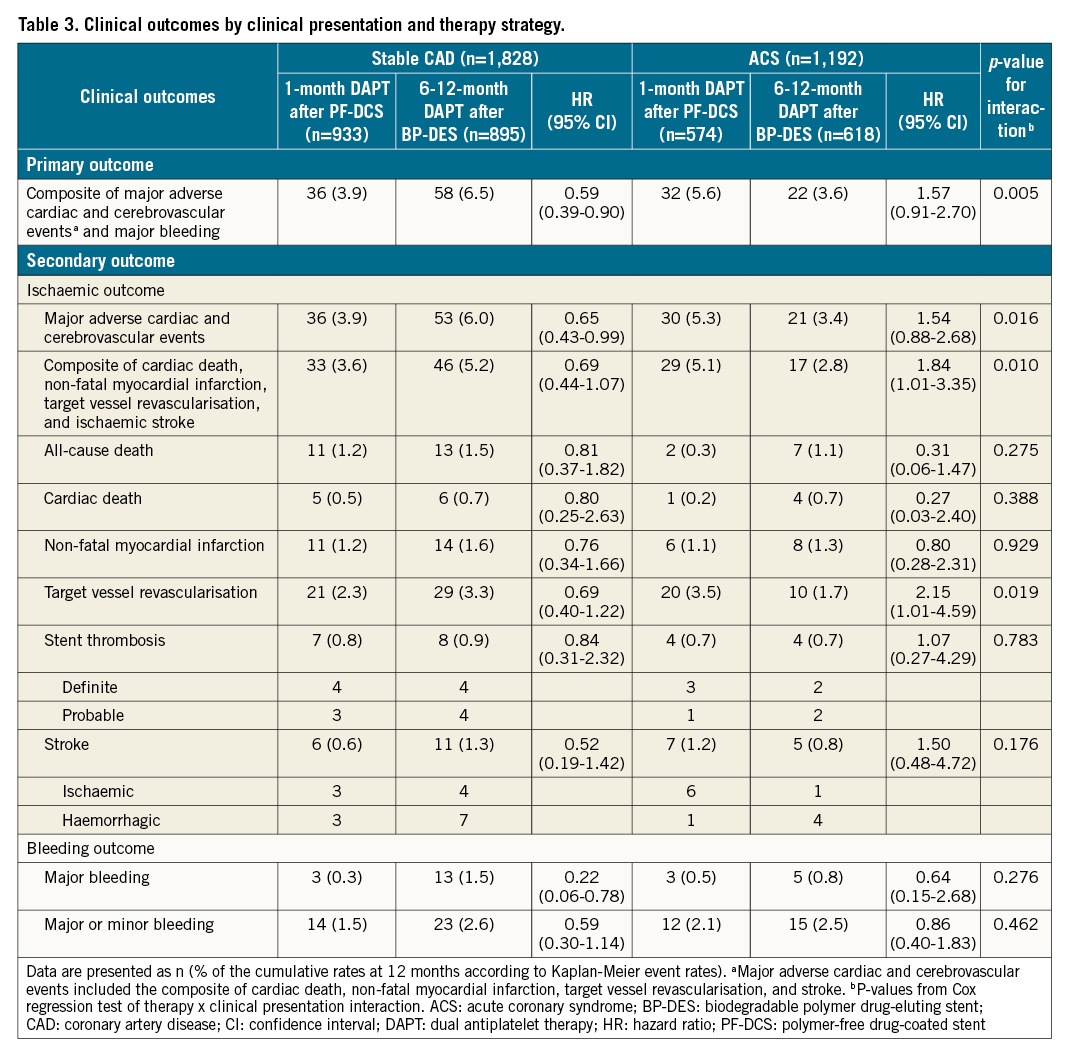
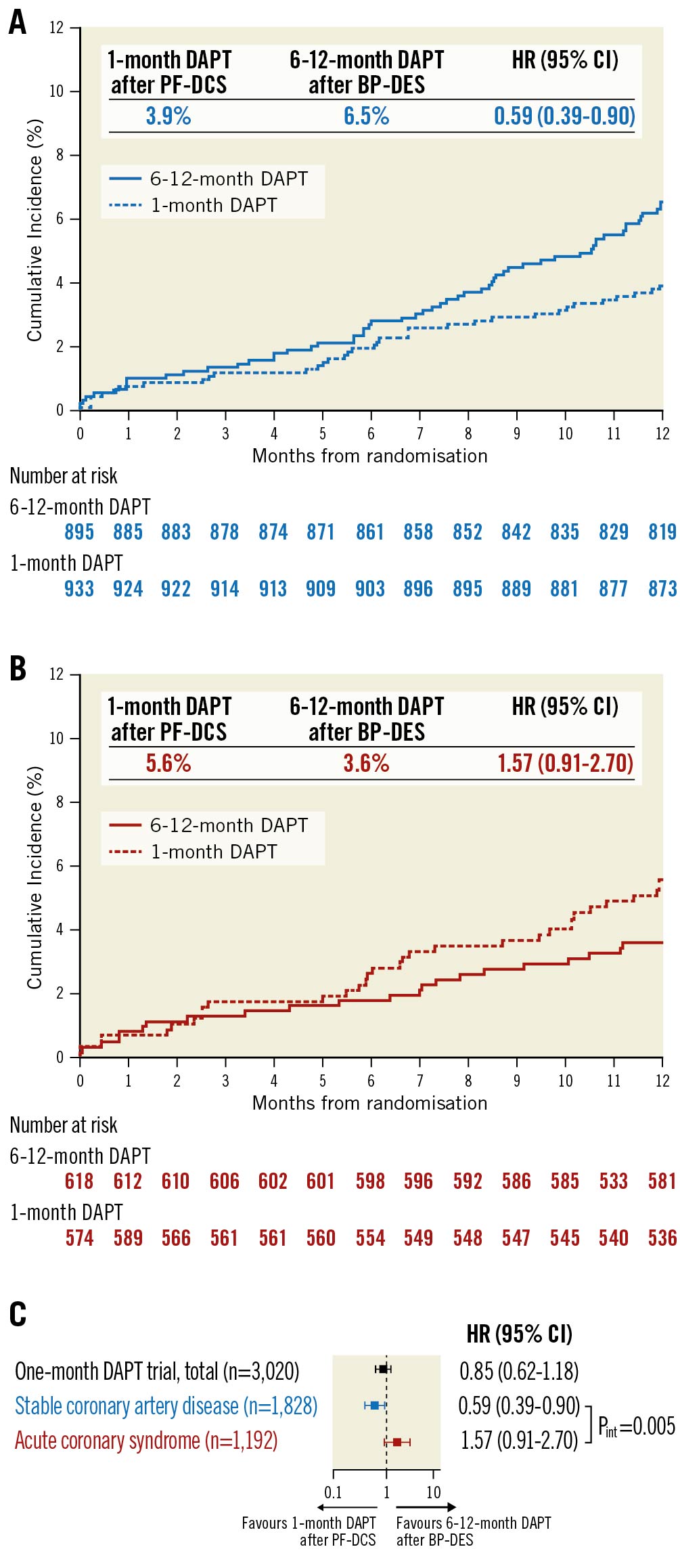
Figure 2. Time-to-event curves and risk for primary outcome by clinical presentation and therapy strategy. Kaplan-Meier survival curves for primary outcome in patients with A) Stable CAD, and B) ACS. C). Relative risk for primary outcome by clinical presentation and therapy strategy. Pint indicates p-value from Cox regression test of therapy × clinical presentation interaction. ACS: acute coronary syndrome; BP-DES: biodegradable polymer drug-eluting stent; CAD: coronary artery disease; CI: confidence interval; DAPT: dual antiplatelet therapy; HR: hazard ratio; PF-DCS: polymer-free drug-coated stent
Additional analyses
In prespecified 1-month landmark analyses, in patients with stable CAD, 1-month DAPT after PF-DCS resulted in lower rates of the primary outcome compared to 6-12-month DAPT after BP-DES (3.2% vs 5.6%; HR 0.56, 95% CI: 0.36-0.89; p=0.013) (Figure 3A). However, in patients with ACS, the rates of the primary outcome were not significantly different between the two therapy groups (5.0% vs 2.8%; HR 1.77, 95% CI: 0.97-3.24; p=0.059) (Figure 3B) and a significant interaction was observed between therapy and clinical presentation, indicating a different therapeutic effect of 1-month DAPT after PF-DCS according to clinical presentation (Pint=0.003) (Figure 3C). A significant interaction was observed in MACCE (Pint=0.009), but not in major bleeding (Pint=0.190) (Supplementary Figure 1A, Supplementary Figure 1B). Similarly, results for the exploratory analyses were consistent after excluding the 88 patients with acute myocardial infarction at index PCI. A significant interaction was observed between therapy and clinical presentation regarding the primary outcome, indicating different therapeutic effects of 1-month DAPT after PF-DCS according to clinical presentation (Pint=0.003) (Supplementary Figure 2A). Further, a significant interaction was observed in MACCE (Pint=0.010), but not in major bleeding (Pint=0.278) (Supplementary Figure 2B, Supplementary Figure 2C). Detailed analyses for clinical outcomes regarding the 1-month landmark and after excluding the patients presenting with acute myocardial infarction are presented in Supplementary Table 1 and Supplementary Table 2.
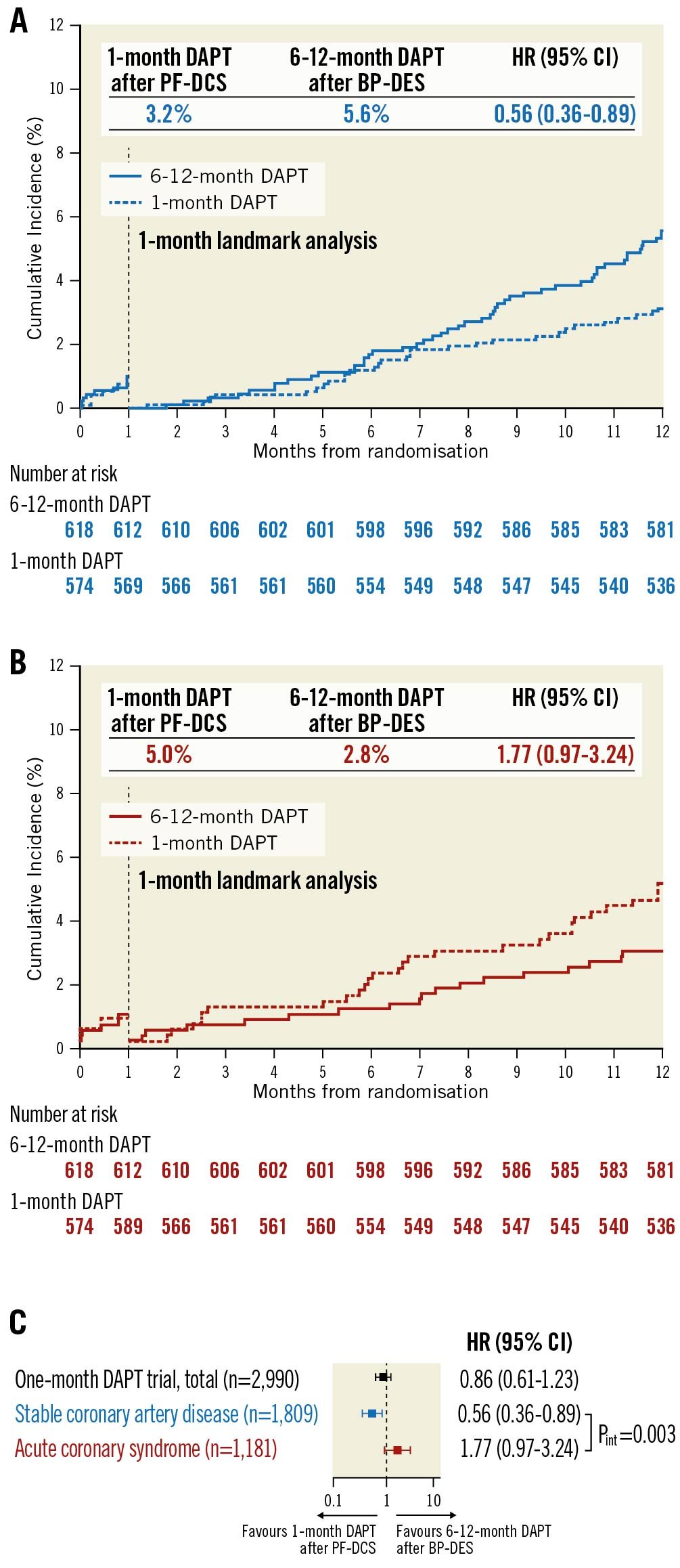
Figure 3. Time-to-event curves and risk for primary outcome by clinical presentation and therapy strategy in 1-month landmark analyses. Kaplan-Meier survival curves for primary outcome in patients with A) Stable CAD, and B) ACS. C) Relative risk for primary outcome by clinical presentation and therapy strategy. Pint indicates p-value from Cox regression test of therapy × clinical presentation interaction. ACS: acute coronary syndrome; BP-DES: biodegradable polymer drug-eluting stent; CAD: coronary artery disease; CI: confidence interval; DAPT: dual-antiplatelet therapy; HR: hazard ratio; PF-DCS: polymer-free drug-coated stent
Discussion
The main findings of this post hoc analysis of the One-Month DAPT trial were as follows (Central illustration): 1) in patients undergoing PCI for non-complex lesions, the effects of 1-month DAPT after PF-DCS vs 6-12-month DAPT after BP-DES regarding the primary outcome and MACCE differed according to clinical presentation, with 1-month DAPT after PF-DCS resulting in lower rates of the primary outcome and MACCE in patients with stable CAD, but presenting no significant difference in rates of the primary outcome and MACCE in patients with ACS; 2) the effects of 1-month DAPT after PF-DCS regarding major bleeding were similar, irrespective of clinical presentation; and 3) results were consistent in 1-month landmark analyses or after excluding the patients presenting with acute myocardial infarction. Overall, compared to 6-12-month DAPT followed by aspirin monotherapy after BP-DES implantation, 1-month DAPT followed by aspirin monotherapy after PF-DCS implantation may be safe for patients with stable CAD but not for those with ACS.
Central illustration. Impact of 1-month DAPT followed by aspirin monotherapy after drug-eluting stent implantation according to clinical presentation. The present post hoc analysis of the One-Month DAPT trial investigated the impact of 1-month DAPT followed by aspirin monotherapy after PF-DCS implantation vs 6-12-month DAPT followed by aspirin monotherapy after BP-DES implantation in patients with stable CAD, compared to those with ACS. The benefits of 1-month DAPT after PF-DCS were present in patients with stable CAD, but not in those with ACS regarding primary (composite of ischaemic and bleeding) and ischaemic outcomes, and a significant interaction was observed between therapy and clinical presentation. There was no heterogeneity in the effects of 1-month DAPT after PF-DCS regarding bleeding outcomes by clinical presentation. ACS: acute coronary syndrome; BP-DES: biodegradable polymer drug-eluting stent; CAD: coronary artery disease; CI: confidence interval; DAPT: dual antiplatelet therapy; HR: hazard ratio; MACCE: major adverse cardiac and cerebrovascular events; PCI: percutaneous coronary intervention; PF-DCS: polymer-free drug-coated stent
The use of a DES in patients with coronary artery disease showed superior clinical outcomes compared to a bare metal stent; however, maintaining DAPT is important after DES implantation to prevent ischaemic events12320. However, major concerns have been raised about an increased risk of bleeding events as well as higher costs, non-compliance, and inconvenience due to prolonged DAPT. Diverse experimental antiplatelet therapy strategies have been proposed with short-term DAPT followed by single antiplatelet therapy after DES implantation4567891021. In addition, with the strategy of DAPT duration maximally shortened to 1 month, the PF-DCS showed superior clinical outcomes compared to a bare metal stent; however, the focus was only on patients with high bleeding risk and single antiplatelet therapy followed by DAPT that was not limited to aspirin8. In contrast, the One-Month DAPT trial showed the non-inferiority of 1-month DAPT after PF-DCS compared to 6-12-month DAPT after BP-DES in patients undergoing PCI for non-complex lesions. Furthermore, the trial was not restricted to those with high bleeding risk and single antiplatelet therapy followed by DAPT that was limited to aspirin in both groups, and it included 1,828 (60.5%) patients with stable CAD and 1,192 (39.5%) patients with ACS.
In general, a shorter duration of DAPT after DES implantation is recommended in patients with stable CAD than in patients with ACS12312. However, the effects of experimental antiplatelet therapy strategy with short-term DAPT followed by single antiplatelet therapy according to clinical presentation are not clearly elucidated. In the STOPDAPT-2 trial, which evaluated the impact of an experimental antiplatelet therapy strategy (1-month DAPT followed by clopidogrel monotherapy) vs a conventional strategy (12-month DAPT) after cobalt-chromium everolimus-eluting stent implantation, the experimental strategy was superior for the primary (composite of ischaemic and bleeding) and bleeding (TIMI major or minor) outcomes, whereas it was comparable for ischaemic outcomes (cardiovascular death, myocardial infarction, stroke, or definite stent thrombosis)4. While no significant interaction between therapy and clinical presentation was observed regarding the primary outcome (Pint=0.64), the interaction regarding ischaemic or bleeding outcome was not addressed4. In the GLOBAL-LEADERS trial, which evaluated the impact of an experimental antiplatelet therapy strategy (1-month DAPT followed by 23 months of ticagrelor monotherapy) vs a conventional strategy (12-month DAPT followed by 12 months aspirin monotherapy) after biodegradable polymer-based biolimus A9-eluting stent implantation, the two strategies were comparable in ischaemic (all-cause death or new Q-wave myocardial infarction) and bleeding (Bleeding Academic Research Consortium [BARC] type 3 or 5) outcomes6. While no significant interaction between therapy and clinical presentation was observed regarding ischaemic outcomes (Pint=0.93), a significant interaction was observed regarding bleeding outcomes (Pint=0.007) with a benefit of experimental strategy present in patients with ACS (HR 0.73, 95% CI: 0.54-0.98; p=0.037), but not in stable CAD (HR 1.32, 95% CI: 0.97-1.81; p=0.081)6. Only the TWILIGHT trial, which examined the impact of an experimental antiplatelet therapy strategy (3-month DAPT followed by ticagrelor monotherapy) vs a conventional strategy (12-month DAPT) after locally approved DES implantation in patients with high-ischaemic or high bleeding risk, conducted a subgroup analysis according to clinical presentation522. A significant interaction between therapy and clinical presentation was observed regarding bleeding outcomes (BARC type 2, 3, or 5; Pint=0.03) with the benefit of the experimental strategy more pronounced in patients with ACS (HR 0.47, 95% CI: 0.36-0.61; p<0.001) compared to stable CAD (HR 0.76, 95% CI: 0.54-1.06; p=0.011). However, no significant interaction was observed regarding ischaemic outcomes (all-cause death, myocardial infarction, or stroke; Pint=0.96) and similar rates were observed between the two strategies, irrespective of clinical presentation22. Nevertheless, the interaction between therapy and clinical presentation regarding the composite of ischaemic and bleeding outcomes was not evaluated in either the GLOBAL-LEADERS or the TWILIGHT trial. In contrast, in the One-Month DAPT trial which specifically focused on patients undergoing PCI for non-complex lesions, the composite of ischaemic and bleeding outcomes was evaluated with DAPT duration shortened to 1 month followed by P2Y12 inhibitor discontinuation instead of aspirin discontinuation, regarding antiplatelet therapy, and only PF-DCS was used regarding DES in the experimental strategy13. In this post hoc analysis according to clinical presentation, a significant interaction between therapy and clinical presentation was observed regarding composite outcomes with a benefit of 1-month DAPT after PF-DCS present in patients with stable CAD, but not in ACS. The results were similar for ischaemic outcomes, but not for bleeding outcomes. These findings were also confirmed in 1-month landmark analyses or after excluding the patients presenting with acute myocardial infarction. Taken together, in patients undergoing PCI for non-complex lesions, although 1-month DAPT followed by aspirin monotherapy after PF-DCS implantation might not be sufficient to find a balance between ischaemic and bleeding risks in patients with ACS, it might be considered a reasonable therapeutic approach in patients with stable CAD. Our findings are in line with a previous meta-analysis that included relatively low-ischaemic risk patients treated with DES and demonstrated different effects of short-term DAPT according to clinical presentation23. Compared to prolonged DAPT, short-term DAPT appeared to be safe in patients with stable CAD, whereas it was associated with higher ischaemic risk in patients with ACS23. Short-term DAPT was associated with lower bleeding risk regardless of clinical presentation23. Similarly, the recently published STOPDAPT-2 ACS trial failed to show non-inferiority of 1-2-month DAPT followed by clopidogrel monotherapy compared to 12-month DAPT regarding the composite of ischaemic and bleeding outcomes, due to numerically higher incidence of ischaemic events in spite of lower incidence of bleeding events in ACS patients undergoing PCI11.
In this study, the benefit of short-term DAPT in patients with stable CAD was not only due to a decrease in bleeding events, but also a decrease in ischaemic events. A similar trend of decreased ischaemic events along with decreased bleeding events with short-term DAPT was also observed in previous studies of STOPDAPT-2 and a secondary analysis of the TWILIGHT trial regarding the diabetes mellitus subgroup424. These findings may imply that bleeding and ischaemic events are not independent; instead, they are interlinked events. This interlinked feature between bleeding and ischaemic events is also in line with previous studies which demonstrated that high bleeding risk based on the Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy (PRECISE-DAPT) score or the Academic Research Consortium for high bleeding risk (ARC-HBR) criteria were associated with higher bleeding events as well as ischaemic events when validated for a large unselected cohort of the Bern PCI registry2526. In this respect, as found in patients with stable CAD undergoing PCI for non-complex lesions in the One-Month DAPT trial, the strategy of 1-month DAPT followed by aspirin monotherapy which primarily aimed to decrease bleeding events may also have been beneficial in decreasing ischaemic events. However, even in patients undergoing PCI for non-complex lesions, those presenting with ACS may require a longer duration of DAPT or more potent single antiplatelet agents after a shorter duration of DAPT compared to those with stable CAD123.
Study limitations
This study has several limitations. First, the therapy strategies used in the One-Month DAPT trial consisted of different antiplatelet therapies combined with different DES between the two groups. Although this may be distinct from previous trials regarding the experimental strategy, a careful interpretation of our findings is required. Second, randomisation was not stratified by clinical presentation in the One-Month DAPT trial and this might have contributed to imbalances in the number of patients allocated to each therapy group within the patients with stable CAD and ACS. Third, although stable CAD or ACS subgroups were not individually powered to yield conclusions on the effect of 1-month DAPT, with a low proportion of patients with acute myocardial infarction, a significant interaction was observed between therapy and clinical presentation regarding the primary outcome. Fourth, the general application of our findings to patients undergoing PCI needs caution since the study was primarily based on patients with non-complex lesions. Therefore, our findings need to be considered only as hypothesis-generating and warrant further prospective confirmation.
Conclusions
In patients undergoing PCI for non-complex lesions, the benefits of 1-month DAPT followed by aspirin monotherapy after PF-DCS implantation compared to 6-12-month DAPT followed by aspirin monotherapy after BP-DES implantation, with respect to the composite of ischaemic and bleeding outcomes, were different according to clinical presentation. The benefits were present in patients with stable CAD, but not in those with ACS. Given that this is a post hoc analysis based on the One-Month DAPT trial, current findings should be considered as hypothesis-generating and require further prospective trials.
Impact on daily practice
After PCI, it is necessary to determine the appropriate minimal duration of DAPT to achieve a balance between bleeding and ischaemic risks, which may differ according to clinical presentation. In patients undergoing percutaneous coronary intervention for non-complex lesions, the benefits of 1-month DAPT followed by aspirin monotherapy after PF-DCS implantation regarding a composite of ischaemic and bleeding outcomes were different according to clinical presentation; the benefits were present in patients with stable CAD, but not in those with ACS. However, these findings of a post hoc analysis need to be considered as hypothesis-generating.
Funding
This work was supported by the Cardiovascular Research Center (Seoul, Republic of Korea) and funded by grants from DIO (Republic of Korea), Cardinal Health Korea (South Korea), and Terumo Corporation (Tokyo, Japan). No funder/sponsor had any role in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

