
Thirteen years ago, the ELUTES trial first suggested that paclitaxel-coated stents might reduce restenosis without requiring a polymer1. However, further evaluation of similar devices was disappointing, so that the first permanent polymer-coated drug-eluting stents (DES) became the early accepted standard2-4. Over time, however, late thrombotic complications became apparent and were largely attributed to the polymer coatings5. Prolonged or indefinite dual antiplatelet therapy (DAPT) was then considered necessary by many until improved permanent and biodegradable polymers were shown to be safer, allowing the pendulum to begin slowly to swing towards less aggressive antithrombotic regimens6. More recently, thanks to better drugs and improved platforms7,8, polymer-free stents have made a comeback, together with the potential for much shorter courses of DAPT. Indeed, in 2015, the LEADERS FREE trial documented that, with only one month of DAPT, a polymer-free drug-coated stent (PF-DCS) eluting Biolimus A9™ (Biosensors Europe, Morges, Switzerland) was both safer and more effective than a bare metal stent in patients considered at high bleeding risk (HBR)9. Several questions remain, however, and two interesting publications in the current issue of EuroIntervention help to broaden our knowledge base10,11.
Sardella et al report on a large multicentre Italian registry of 1,100 all-comer patients treated with a Biolimus A9 (BA9) PF-DCS and followed for one year10. DAPT duration and choice of P2Y12 inhibitor were left to the discretion of the operators. The primary endpoint of cardiac death, myocardial infarction (MI) and definite or probable stent thrombosis (ST) occurred in 4.1% of patients. Based on a CRUSADE score of >40, the authors considered a subgroup of their patients to be HBR. The primary endpoint rate was nearly three times higher in these patients (9.1%) than in the non-HBR group (3.2%).
Despite the limitations inherent to all registries, these findings are significant since they document excellent efficacy (TLR 1.2%) and good safety (stent thrombosis 1.1%) in routine clinical practice, in line with the suggested objective performance targets proposed by the ESC/EAPCI Task Force for coronary stent evaluation12. The 1.1% rate of ST is broadly comparable to that reported with other contemporary DES and, despite the fact that all events occurred while patients were on DAPT, it is important to note that the ST rates were largely driven by patients with high CRUSADE scores13-15. This further confirms that the comorbidities associated with an increased bleeding risk very often also carry an increased thrombotic risk. Patient characteristics thus remains a critical driver of adverse events, as illustrated in Figure 1. Regarding the stent platform, the current BA9 PF-DCS is made of stainless steel with a strut thickness of 120 μm. Bangalore et al have recently shown that ultra-thin strut metallic DES, when compared to thicker strut DES, are associated with a lower rate of MI (mostly periprocedural) and with a non-significant trend towards a lower rate of stent thrombosis16. The LEADERS FREE III trial (NCT03118895) is currently evaluating a polymer-free BA9-eluting cobalt-chromium stent with struts of 84 μm thickness. If the results can replicate the safety and efficacy of the stainless steel platform, it should join the group of best-in-class workhorse stents with the added benefit of maximal documented flexibility in terms of DAPT duration. The SENIOR trial has recently suggested that a bioabsorbable polymer DES was as safe but more effective than a BMS for elderly patients treated with one or six months of DAPT17. Several other large trials, such as ONYX ONE (NCT03344653) and MASTER DAPT (NCT03023020), are ongoing to assess whether other DES, with either permanent or bioabsorbable polymers, can also be used safely with an ultra-short one-month course of DAPT.
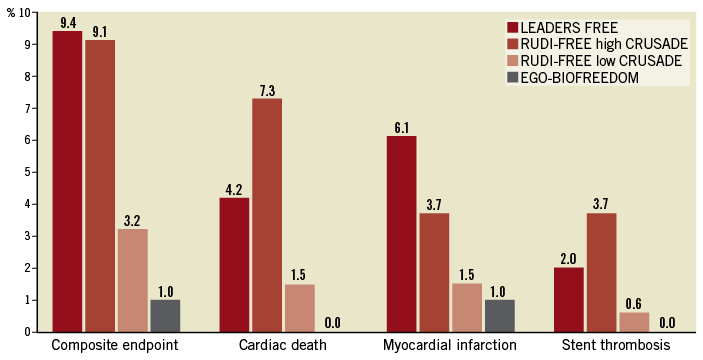
Figure 1. Rates of the composite endpoint (cardiac death, myocardial infarction or stent thrombosis) and its components in three trials (RUDI-FREE, EGO-BIOFREEDOM and LEADERS FREE9-11). The results for RUDI-FREE patients are reported separately for patients with a high (>40) baseline CRUSADE score, and those with a lower score.
Two limitations of the RUDI-FREE study deserve to be mentioned: the lack of recorded and adjudicated bleeding events is a weakness shared with the majority of stent registries, and the choice of the CRUSADE score to define bleeding risk is debatable. The authors considered a score of 40 or above to define HBR, but it should be stressed that CRUSADE was designed specifically to predict only in-hospital bleeding for patients not on oral anticoagulation (OAC) and presenting with non-ST-segment elevation myocardial infarction (NSTEMI). Forty-six patients (30.1%) in the high CRUSADE score group were discharged on OAC, but so were 51 patients (5.4%) in the lower score group, although the latter would be considered as HBR by most interventionists. In any case, the CRUSADE score appears to have had very little impact on the physicians caring for these patients, since at one-year follow-up 64% of patients with a high score were still on DAPT. Had they been available, the more recent DAPT and PRECISE DAPT scores might have been better suited to assess the bleeding risk after discharge, although it should be noted that both those scores selected mostly lower-risk patients and also excluded those on OAC18,19.
Lee et al evaluated the strut coverage of the BA9 PF-DCS by serial staggered optical coherence tomography (OCT) measurements in 100 patients11. Their main findings are that the one-month median coverage is excellent at 85.8%, and the nine-month strut coverage is in the same range as that reported for several DES with permanent or biodegradable polymer coatings20. Also important is the angiographic late loss of 0.21±0.30 mm, which is in keeping with other values previously reported, and confirms that the stent effectively inhibits neointimal proliferation despite the relatively rapid drug elution7,21,22. Importantly, the data lend mechanistic support to the concept that very short DAPT may be an appropriate option with this device when the estimated bleeding risk makes it necessary.
While perhaps not always acceptable for all patients (three invasive imaging procedures over a nine-month period), the sequential acquisition of OCT data permits a unique understanding of the healing process after stent implantation. Lee at al have applied it to several different stents, thus making comparisons tempting. In the present series, median strut coverage was 87% in 20 patients at two months. Using the same technique, definitions and time point, the median strut coverage was 77.1% with an endothelial progenitor cell-capturing sirolimus DES, and the mean strut coverage was 87.2±8.1% with a biodegradable polymer BA9 DES, while it was 91.6±5.9% with a zotarolimus permanent polymer DES23,24. Using different OCT criteria, another biodegradable polymer-coated sirolimus-eluting stent was 89±9.9% covered at two months25.
It would therefore appear that several stents, polymer-free or not, when implanted with OCT guidance in patients without STEMI for relatively simple lesions, allow good early strut coverage. Very early after stent implantation, however, the clinical value of this parameter remains uncertain since no agreement exists today on the degree of strut coverage that allows safe DAPT discontinuation, and there is considerable variability of early coverage rates observed in individual patients26. Also, the true sensitivity and specificity of OCT strut “coverage” to predict histological endothelialisation is unknown, since very thin endothelium can be missed and a visible non-cellular layer may be mistaken for endothelium. The complexity of the issue is further increased by the fact that many other factors, probably often more important than early strut coverage, play a role in stent thrombogenicity, such as pre-existing thrombus, stent malapposition, underdeployment, overlap or fracture, as well as total length and minimal stent diameter, to mention just the most important. Only by meticulously accumulating high-quality intraluminal imaging data such as those collected by Lee et al will we hopefully learn to differentiate what really matters from what perhaps does not. Ultimately, late events related to neoatherogenesis may determine the comparative long-term safety of PF-DCS vs. polymer-coated DES. In this respect, acquisition of late and very late OCT images will also remain of major interest27. While we must wait for the results of face-to-face comparisons such as the Onyx ONE trial to know whether a polymer-coated DES is as safe and effective as a BA9 PF-DCS with ultra-short DAPT for HBR patients, the two trials published in this edition of EuroIntervention suggest that, in many ways, a PF-DCS with guideline-recommended DAPT duration behaves and can probably be considered as a workhorse stent for all-comer patients10,11.
Conflict of interest statement
P. Urban is a consultant to Biosensors. B. Chevalier is a consultant to Biotronik, Cordis, Medtronic and Terumo.

