
Abstract
Aims: Polymer-free biolimus-eluting stents (PF-BES) have been shown to be superior to bare metal stents in high bleeding risk (HBR) patients treated with one-month dual antiplatelet therapy (DAPT). However, limited evidence is available on PF-BES in non-HBR patients. We aimed to evaluate the safety and efficacy of PF-BES in all-comer patients undergoing percutaneous coronary intervention (PCI).
Methods and results: Patients with stable coronary artery disease or acute coronary syndromes (ACS) undergoing PCI with PF-BES in routine clinical practice were included in a multicentre, prospective registry. DAPT duration was left to the discretion of the operator. The primary endpoint was the composite of cardiovascular death, myocardial infarction (MI), and definite/probable stent thrombosis (ST) at one year. Overall, 1,104 consecutive patients treated with PF-BES were included at 16 Italian centres. Mean age was 68.7±11.2 years, 77.2% of patients were male, 30% had diabetes, 15.1% had chronic kidney disease, and 40.5% had ACS at baseline. Mean CRUSADE score was 24.1±13.1, and 83.7% of patients did not have high bleeding risk features. At one year, the primary endpoint occurred in 4.1% of patients, cardiovascular death in 2.4%, MI in 1.8%, and definite/probable ST in 1.1%. With respect to efficacy, target lesion revascularisation occurred in 1.2% of patients.
Conclusions: This is the first study providing clinical evidence on the use of PF-BES in all-comer patients irrespective of HBR status. Our findings suggest that PF-BES has a favourable safety and efficacy profile in a real-world clinical setting. Further investigation in randomised clinical trials against new-generation DES is warranted.
Abbreviations
ACS: acute coronary syndromes
CAD: coronary artery disease
DAPT: dual antiplatelet therapy
DES: drug-eluting stent
HBR: high bleeding risk
MI: myocardial infarction
PF-BES: polymer-free biolimus-eluting stent
PCI: percutaneous coronary intervention
ST: stent thrombosis
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Drug-eluting stents (DES) have markedly improved clinical outcomes in patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI)1. Over the past 15 years, technological advances in DES technologies have determined a progressive improvement in the safety and efficacy profile of DES2,3. New devices with a more favourable biocompatibility have been shown to reduce the risk of thrombotic events without impairing the antirestenotic efficacy of early-generation DES4.
The polymer-free biolimus-eluting stent (PF-BES) (BioFreedom™; Biosensors Europe SA, Morges, Switzerland) is based on a stainless steel platform with surface modifications from which the antirestenotic agent is directly released without the use of a polymeric carrier. In the large-scale LEADERS FREE trial, PF-BES were shown to improve safety and efficacy as compared with bare metal stents in patients at high bleeding risk (HBR) treated with one-month dual antiplatelet therapy (DAPT)5-8. However, limited evidence is available on the clinical outcomes of PF-BES in non-HBR patients, who represent the majority of patients treated in clinical practice.
The aim of this study was to evaluate the safety and efficacy profile of BF-BES in real-world, all-comer patients with CAD undergoing PCI.
Methods
STUDY DESIGN
Between January 2015 and May 2016, consecutive patients with CAD undergoing PCI with PF-BES implantation at 16 Italian centres were included in the PolymeR free biolimUs eluting stent implantation in all-comers population: analysis of DAPT cessation and clinical outcome after BioFREEdom stent implantation (RUDI-FREE) observational, multicentre, prospective, single-arm study (ClinicalTrials.gov identifier: NCT02858739). Inclusion criteria were broad and reflected routine clinical practice, including patients with stable CAD as well as acute coronary syndromes (ACS) – with or without ST-segment elevation. The study complied with the Declaration of Helsinki and was approved by local ethics committees. Each patient provided informed consent for participation in the study.
Further details on inclusion/exclusion criteria, medical regimen, study device features, data collection, follow-up procedures and the event adjudication process are provided in the Supplementary Appendix.
ENDPOINTS
The primary endpoint was a composite endpoint of cardiovascular death, myocardial infarction (MI), and definite or probable stent thrombosis (ST) at 12 months. Secondary endpoints are detailed in the Supplementary Appendix.
STATISTICAL ANALYSIS
A detailed description of analyses performed is provided in the Supplementary Appendix.
Results
From January 2015 to June 2016, out of approximately 7,500 patients undergoing PCI with stent implantation, a total of 1,104 patients consecutively undergoing PCI with PF-BES in routine clinical practice were included at 16 Italian centres. The baseline clinical characteristics of the included patients reflected the real-world nature of the study. Mean age was 68.7±11.2 years, 22.7% of patients were female, 30% had diabetes, and 15.2% had chronic kidney disease. Mean left ventricular ejection fraction was 49.7±10.8%. With respect to indications for PCI, 59.5% of patients had stable CAD, 27.3% had non-ST-elevation ACS, and 13.2% had ST-elevation MI. Mean CRUSADE score was 24.1±13.1, with a total of 164 patients (16.3%) having a CRUSADE score >40 and, therefore, considered at HBR. The baseline clinical characteristics for the overall population and according to HBR status are summarised in Table 1. HBR patients were characterised by a higher baseline risk profile in terms of risk factors and comorbid conditions as compared with non-HBR patients.
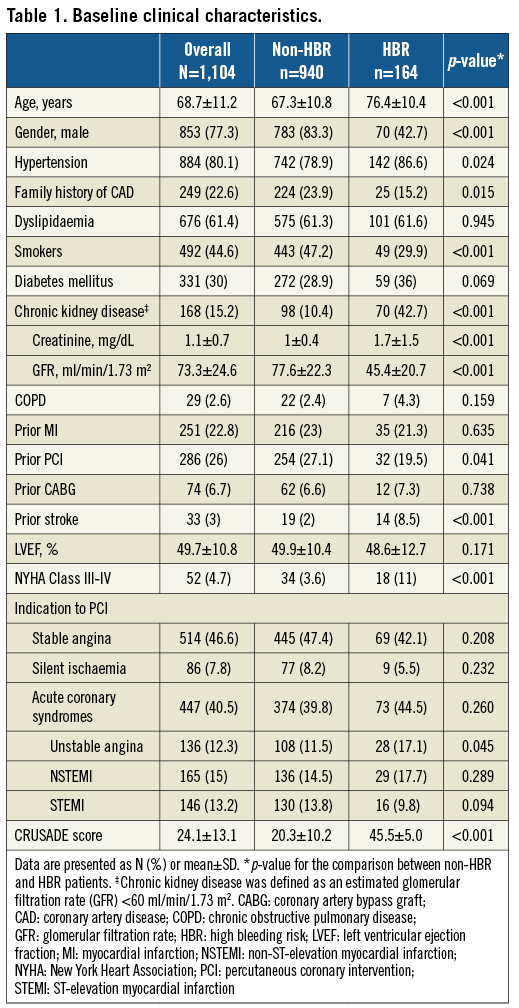
Lesion and procedural characteristics are summarised in Table 2. A total of 1,667 lesions were treated with an average of 1.5 lesions per patient. Lesions were homogeneously distributed among the epicardial vessels, with the majority being located in the left anterior descending artery (42.3%). No significant differences in lesion location were observed between HBR and non-HBR patients. Lesion complexity was consistent with the all-comer profile of the study population. More than half of the treated lesions (56.1%) were type B2/C according to the ACC/AHA classification, 37% were longer than 20 mm, 11% were CTOs, 15.4% were bifurcations, and 10.6% were severely calcified. HBR patients had a higher prevalence of severely calcified (20.4% vs. 8.9%, p<0.001) and long lesions (46% vs. 35.5%, p=0.001) as compared to non-HBR patients.
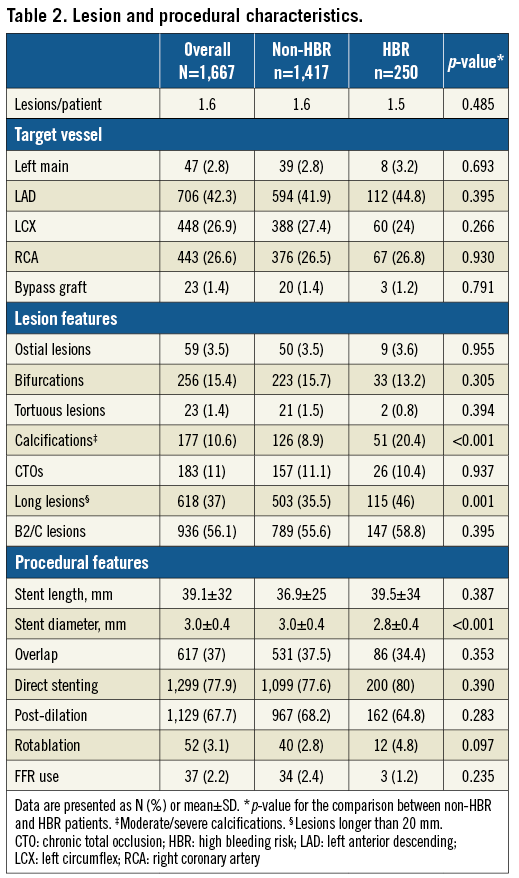
Medical therapy at discharge and at one-year follow-up is summarised in Table 3. DAPT was recommended at discharge for one month in 4.9% of patients, for three months in 4.4% of patients, for six months in 35.8% of patients and for 12 or more months in 55.0% of patients.
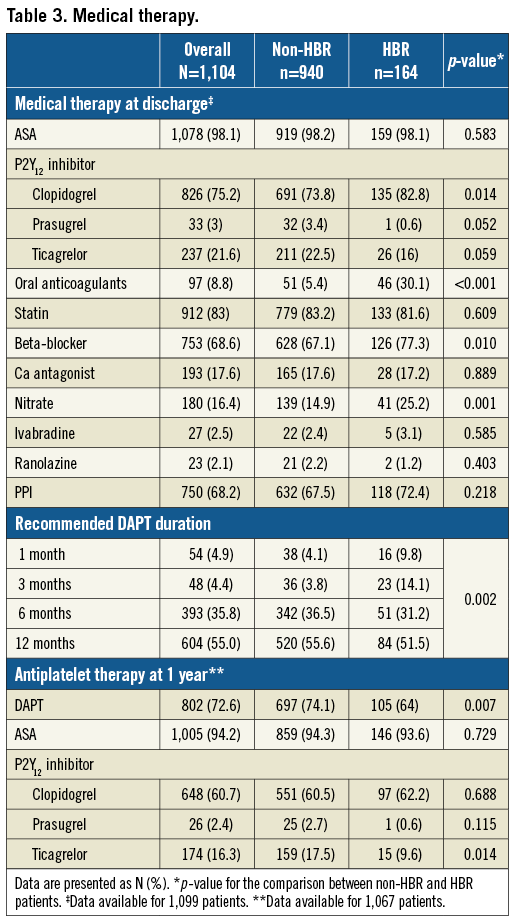
Twelve-month follow-up was completed in 97.2% of patients, with a mean follow-up of 346±29 days. Clinical outcomes are reported in Table 4. Overall, the primary endpoint occurred in 4.1% of patients at one year (Figure 1). With respect to secondary ischaemic endpoints, all-cause death occurred in 3.9% of patients, cardiovascular death in 2.4% of patients, MI in 1.8% of patients, and target vessel myocardial infarction (TV-MI) in 1.0% of patients at one year. In relation to device efficacy, TLR occurred in 1.4% of patients and TVR in 1.8% of patients at one year. In relation to device safety, definite/probable ST occurred in 1.1% of patients and definite ST in 0.4% of patients at one year. Specifically, 12 definite/probable ST events occurred, four of which were definite ST and eight probable ST. All ST events occurred on DAPT. The cumulative incidence of TLR and definite ST up to one year is shown in Figure 2.
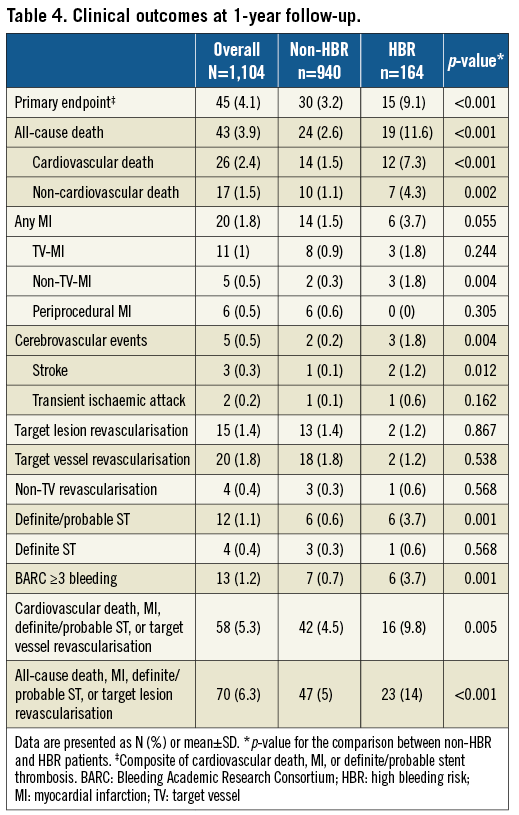
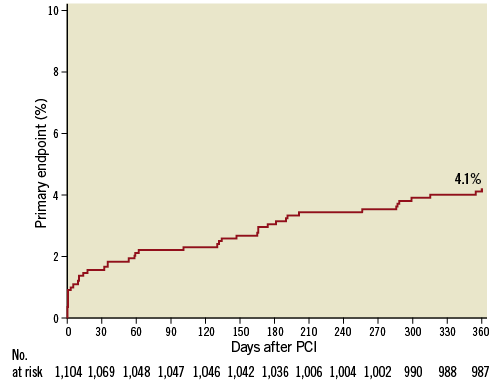
Figure 1. Cumulative incidence of the primary endpoint (composite of cardiovascular death, MI, and definite/probable ST) up to one year.
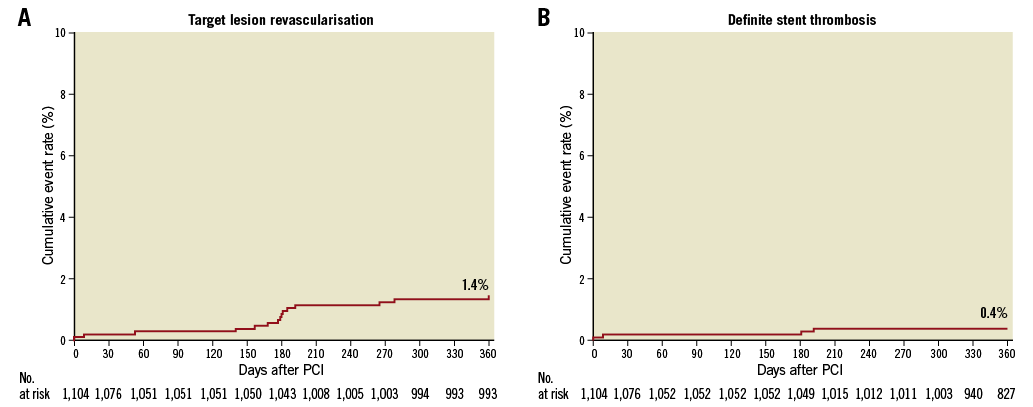
Figure 2. Cumulative incidence of target lesion revascularisation (A) and definite stent thrombosis (B) up to one year.
The primary endpoint cumulative incidence up to one year by HBR status is presented in Figure 3. The primary endpoint occurred more frequently in HBR compared with non-HBR patients (9.1% vs. 3.2%, p<0.001).
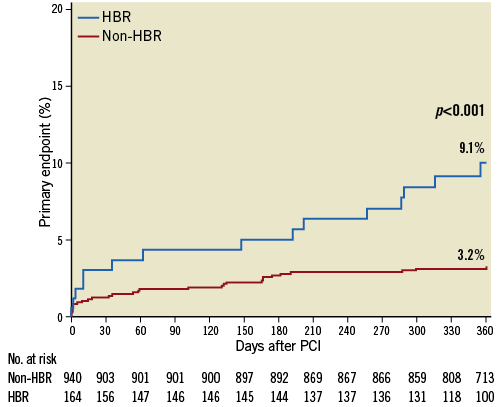
Figure 3. Cumulative incidence of the primary endpoint stratified according to bleeding risk up to one year. HBR: high bleeding risk
Discussion
The present study is the first report providing clinical evidence on the use of PF-BES in real-world all-comer patients. Our findings can be summarised as follows:
1) Clinical outcomes of PF-BES in real-world all-comer patients up to one year are comparable with contemporary new-generation DES.
2) TLR and ST rates are low, indicating an excellent efficacy and safety profile of PF-BES.
3) Clinical outcomes stratified by HBR status support the use of PF-BES in non-HBR patients, in whom PF-BES have not previously been investigated.
Drug-eluting stents have revolutionised the treatment of CAD by markedly improving the clinical outcomes of patients undergoing PCI. Since their introduction, DES technologies have been subject to several iterations with the aim of improving device biocompatibility and, subsequently, efficacy and safety outcomes. In this context, polymer-free DES have been developed based on the hypothesis of improving clinical outcomes by eliminating the inflammatory pro-thrombotic trigger of polymer coatings9-11. Preliminary preclinical evidence in a porcine model showed a lower degree of neointimal proliferation and inflammation at 180 days with PF-BES as compared to early-generation sirolimus-eluting stents12. Moreover, PF-BES were proven to be non-inferior in terms of in-stent late lumen loss at 12 months as compared to early-generation paclitaxel-eluting stents in a small-scale randomised trial including 182 selected low-risk patients with single de novo target lesions in native coronary vessels8. Similarly, two small-scale registries have shown favourable angiographic efficacy of PF-BES in 72 patients with de novo lesions7 and 175 patients with ST-elevation myocardial infarction6. More recently, pivotal clinical evidence on this novel device was provided by the LEADERS FREE trial that directly compared PF-BES with bare metal stents in 2,466 HBR patients treated with a DAPT regimen of one-month duration5. In this trial, PF-BES resulted in being superior to BMS in terms of efficacy and, most importantly, safety. Specifically, patients treated with PF-BES had a lower risk of the primary safety endpoint – a composite of cardiovascular death, MI, and definite/probable ST, corresponding to the primary endpoint of the present study – as compared to patients treated with BMS. Based on the LEADERS FREE findings, PF-BES are currently considered a safe and effective choice for HBR patients undergoing PCI. However, event rates observed in the LEADERS FREE trial were relatively high when compared with event rates associated with the use of contemporary new-generation DES4. Whether this was due to the high-risk profile of HBR patients or to the device performance has been a matter of debate. In the present study, we provide the first evidence on the use of PF-BES in all-comer real-world patients irrespective of bleeding risk. As expected from the study design, the study population was mostly composed of patients without HBR characteristics (83.7% of included patients). In line with previously published all-comer studies, 54.4% of included patients had stable CAD. Of note, however, the anatomical presentation of treated CAD was relatively complex, as indicated by a high prevalence of chronic total occlusions (11%) and B2/C lesions (56.1%). Overall, our findings indicate an excellent safety and efficacy performance of PF-BES in all-comer patients.
DAPT duration was left to the operators’ discretion in the present study. Of note, the study was initiated prior to the publication of the LEADERS FREE trial, which explains the longer than expected average DAPT duration.
Analyses stratified by HBR status further support our findings. The primary endpoint occurred in 9.1% of patients, an event rate that is consistent with the 9.4% observed among patients treated with PF-BES in the LEADERS FREE trial. This confirms the validity of the LEADERS FREE findings in a real-world population and suggests that the relatively high event rates observed in the LEADERS FREE trial could be explained by the intrinsic high-risk nature of HBR patients.
Recently, a European Society of Cardiology (ESC)/European Association for Percutaneous Cardiovascular Interventions (EAPCI) Task Force for coronary stent evaluation has performed a systematic review on the available randomised evidence on coronary stents, providing average rates and ranges of adverse events observed with contemporary new-generation DES4. The same document recommended the use of objective performance criteria based on the available evidence for the evaluation of novel metallic DES. Figure 4 provides an overview of event rates observed with PF-BES among non-HBR all-comer patients in the present study as compared with ranges of adverse event rates observed with contemporary new-generation DES in the systematic review performed by the ESC/EAPCI Task Force. It indicates that PF-BES safety and efficacy performance compares favourably with contemporary new-generation DES.
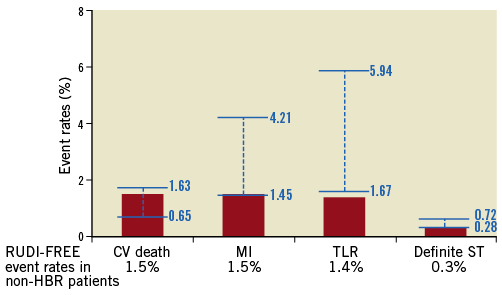
Figure 4. Event rates with polymer-free biolimus-eluting stents in non-HBR all-comer patients (red columns) compared with ranges of adverse event rates (blue bars) observed with contemporary new-generation DES in the systematic review performed by the ESC/EAPCI Task Force for coronary stent evaluation4. CV death: cardiovascular death; MI: myocardial infarction; ST: stent thrombosis; TLR: target lesion revascularisation
As mentioned above, the PF-BES evaluated in the present study is based on a stainless steel stent platform with relatively thick struts (i.e., 112 µm). Contemporary DES are largely based on cobalt-chromium or platinum-chromium alloys which allow thinner strut thickness. Therefore, the favourable performance of the current version of PF-BES is remarkable. Of note, an iterated version of PF-BES based on a cobalt-chromium alloy has been developed and is currently being investigated in a clinical trial (ClinicalTrials.gov identifier: NCT03118895).
Further evidence on the safety and efficacy profile of PF-BES will be provided by ongoing studies such as the SORT-OUT IX trial that is comparing PF-BES with the biodegradable polymer-based sirolimus-eluting Orsiro stent (Biotronik, Berlin, Germany) in all-comer patients (ClinicalTrials.gov identifier: NCT02623140), and the Onyx ONE Study that is comparing BF-BES with the durable polymer-based zotarolimus-eluting Resolute™ stent (Medtronic, Minneapolis, MN, USA) in HBR patients treated with one-month DAPT (ClinicalTrials.gov identifier: NCT03344653).
Limitations
These findings should be interpreted in the light of some limitations. First, the observational design of the study is prone to selection bias. However, the relatively high risk of the included patients is reassuring – as indicated by baseline risk profiles – concerning the real-world all-comer nature of the study population. Noteworthy, mean age (68.7±11.2 years), indication to PCI (ACS in 40.5%, STEMI in 13.2%), and prevalence of risk factors such as diabetes (30%) and chronic kidney disease (15.2%) are comparable with previously published all-comer studies on DES13-16. Second, this study was not powered to evaluate rare adverse events such as ST and TLR. Therefore, the study size does not allow any inferential speculations on the occurrence of ST according to DAPT cessation or HBR status. However, the extremely low ST and TLR event rates are reassuring regarding the safety and efficacy profile of PF-BES in routine clinical practice. Third, the ideal definition of high bleeding risk is a matter of debate. We applied the CRUSADE score to define bleeding risk. We acknowledge the limitation of applying this score; however, we took advantage of having a prospective assessment of this score in all included patients. Finally, the absence of a comparator represents an important limitation. However, it is noteworthy that one-year event rates observed with PF-BES in this study fall within the ranges of adverse event rates observed with contemporary new-generation DES in the systematic review performed by the ESC/EAPCI Task Force on coronary stent evaluation. Therefore, PF-BES performance appears to be comparable to contemporary polymer-based new-generation DES in an all-comer population including HBR as well as non-HBR patients.
Conclusions
The findings of this study suggest that PF-BES has a favourable safety and efficacy profile in a real-world clinical setting irrespective of HBR status. Further investigation in randomised clinical trials against new-generation DES is warranted.
| Impact on daily practice Polymer-free biolimus-eluting stents (PF-BES) have been shown to be superior to bare metal stents in high bleeding risk (HBR) patients treated with one-month dual antiplatelet therapy. However, limited evidence is available on PF-BES in all-comer populations – largely constituted by non-HBR patients. Currently, based on the available evidence, PF-BES are mainly used in HBR patients not compliant with long-term dual antiplatelet therapy. The findings of the present study indicate that PF-BES have a favourable safety and efficacy profile in real-world all-comer patients undergoing PCI. Therefore, PF-BES use should not be limited to HBR patients. |
Funding
The study was sponsored by the Italian Society of Invasive Cardiology (GISE) through an unrestricted grant from Biosensors Europe (Morges, Switzerland).
Conflict of interest statement
G. Sardella has received proctor fees from Edwards Lifesciences, Boston Scientific and Biosensors, and speaker fees from AstraZeneca, Terumo, OrbusNeich, Biosensors, Boston Scientific, Abbott Vascular, Stentys, and Alvimedica. G. Stefanini has received a research grant (to the institution) from Boston Scientific and speaker/consultant fees from B. Braun, Biosensors, and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Details of the RUDI-FREE study.
To read the full content of this article, please download the PDF.

