Abstract
Aims: Previous randomised studies have shown a significant reduction in restenosis when oral rapamycin (OR) is administered to patients undergoing bare metal stent (BMS) implantation. How this regimen compares to drug eluting stents (DES) is unknown.
Methods and results: Two-hundred patients with de novo coronary lesions were randomised to treatment with OR plus BMS (100 pts) or with DES (100 pts). OR was given as a bolus of 10 mg per day before PCI followed by daily doses of 3 mg during following 13 days. Primary endpoints were to compare hospital, follow-up and overall cost at one, two, three and five years of follow-up. The secondary endpoints included death, myocardial infarction (MI) and stroke and were analysed as major adverse cardiovascular events (MACCE). Target vessel (TVR) and target lesion revascularisation (TLR) were independently analysed. Costs included procedural resources, hospitalisation, medications, repeat revascularisation procedures and professional fees. Baseline demographic, clinical and angiographic characteristics were similar. At 18.3±7 months of follow-up, the initial strategy of OR plus BMS resulted in significant cost saving when compared to DES (p=0.0001). TLR rate was 8.2% with DES and 7.0% with OR plus BMS (p=0.84), similarly no differences in TVR rate in both groups was seen (10.6% and 10.5% in OR and DES group respectively, p=0.86). Non-inferiority testing, determined that DES therapy failed to be cost saving compared to OR in all possible cost scenarios.
Conclusions: A strategy of OR plus BMS is cost saving compared to DES in patients undergoing PCI for de novo coronary lesions.
Abbreviations and acronysm
PCI: Percutaneous coronary intervention
DES: Drug eluting stents
ORAR: Oral Rapamycin in ARgentina Study
OR: Oral rapamycin
AMI: Acute myocardial infarction
TVR: Target vessel revascularisation
TLR:Target lesion revascularisation
Introduction
In recent years, drug eluting stents (DES) have been associated with significant reductions of in-stent restenosis among patients undergoing percutaneous coronary interventions (PCI) in de novo coronary lesions1-5. Despite the fact that all these devices have shown that the stent polymeric coating is a good solution for storing drug and defining a release mechanism, several publications have raised concerns about long-term safety issues such as inducing chronic inflammation in the coronary artery and the potential risk for late stent thrombosis6-11.
Several preclinical studies have demonstrated the ability of systemically administered sirolimus in reducing neointimal proliferation occurring after vascular injury12,13 and recently observational and randomised clinical studies have been reported with oral administration14-18.
The ORAR II (Oral Rapamycin in ARgentina) randomised trial showed a significant reduction in angiographic and clinical restenosis in patients treated with oral rapamycin (OR) plus bare metal stents (BMS) as compared to BMS alone19.
The purpose of the present study was to compare the cost associated with an oral sirolimus plus BMS strategy for PCI with a DES strategy and to evaluate the relative safety and efficacy of both treatment approaches.
Materials and methods
Patient population and study design
From January 2006 to September 2007, patients undergoing coronary stent implantation at the catheterisation laboratories of three centres in Buenos Aires, Argentina (Sanatorio Otamendi, Las Lomas and Clinica IMA) were evaluated and those that met the study clinical inclusion criteria were asked to consent to the study. Patients were randomly assigned to either OR plus BMS or the DES arm. Those patients who subsequently failed to meet the pre-specified angiographic criteria for inclusion, or met any of the angiographic exclusion criteria, were dropped from the study at this point. Additionally, patients with clinical and angiographic criteria for randomisation, but unable for PCI with DES deployment, were not randomised (Figure 1).
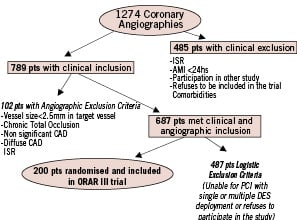
Figure 1. Patient population and study design of ORAR III.
ISR: In-stent restenosis; CAD: coronary artery disease; AMI: acute myocardial infarction
The inclusion criteria were: clinical indication for myocardial revascularisation, age greater than 18 years, de novo severe stenosis suitable for stenting (≥70% visual estimation), reference vessel size between 2.5 mm and 4.0 mm by visual estimation and the fact that these patients were also suitable for coronary bypass surgery (CABG).
The exclusion criteria were: an acute myocardial infarction (MI) in the preceding 24 hours, in-stent restenosis, previous PCI in the last six months, chronic total occlusion of the target vessel, rapamycin allergy, clopidogrel or aspirin intolerance, significant bleeding in the last six months, stroke or transient ischaemic attack in the last 12 months, major blood dyscrasias including thrombocytopenia, patients with poorly controlled dyslipidaemia, participation in another trial which did not allow long term clopidogrel treatment, short life expectancy, or patients with infectious diseases.
The protocol of this non-industry sponsored study was approved by the Ethics Committee of the participating centres and by the Argentina National Regulatory Agency for Drug, Food and Medical Technology (ANMAT). During the study, an Independent Safety Clinical Events Monitoring Committee, whose members were blinded to the patient’s assigned treatment group, adjudicated the clinical adverse events. The study was conducted according to the principles of the Declaration of Helsinki, and all the patients signed a written informed consent for participation in this trial. The Cardiovascular Research Centre, a nonprofit organisation which conducted previous ORAR studies, was in charge of the trial management, the accuracy of the data analyses, and the completeness of the material reported.
Randomisation, medication and coronary procedures
All eligible patients were randomised to OR plus BMS or DES. The randomisation process in each centre was performed in a blind manner from the coordinating centre with the use of an internet system containing a block randomisation sequence for each participating centre.
In the OR arm, patients received a loading dose of 10 mg the day before stent implantation, followed by 3 mg per day for a total of 14 days. Diltiazem sustained release 180 mg a day, was added to sirolimus regimen in order to achieve higher sirolimus blood concentrations17.
Blood samples were drawn at baseline and 30 days after PCI to assess serum creatinine, cholesterol, triglycerides, red and white blood cell and platelet counts. Taking into account previous experiences with the oral administration15,18-19, sirolimus blood concentration was not measure at this time.
A clinical interview after hospital discharge was required twice during the first month of treatment and monthly thereafter up to six months with additional interviews at nine months and one year after the intervention.
Coronary angiography after PCI was performed only on the basis of a clinical indication.
Percutaneous coronary intervention and stent procedure
PCI was performed using standard techniques described elsewhere17. In the BMS group, patients received any of the following stents: Multilink (Boston Scientific Corporation, Natick, MA, USA), Driver (Medtronic Vascular, Santa Rosa, CA, USA), Liberté (Boston Scientific Corporation, Natick, MA, USA) or Eucastsflex (Eucatech AG, Rheinfelden, Germany). In the DES group, patients received one of the four commercially available stents: Taxus (Boston Scientific Corporation, Natick, MA, USA), Endeavour (Medtronic Vascular, Santa Rosa, CA, USA) Cypher, (Cordis, Warren, NJ, USA) and EucaTax (Eucatech AG, Rheinfelden, Germany). All these stents are commercially available in Argentina and have had approval for clinical use by the regulatory authorities either in the USA or Europe. In the DES group, a BMS was allowed if implanted in a secondary or side branch vessel and a DES was not implanted in the same vessel. Overlapping DES with BMS was not allowed. All patients received 325 mg/day of aspirin indefinitely and clopidogrel as a loading dose of 300 mg on the day of the procedure and 75 mg/day thereafter for one month in the OR arm, and mandatory for one year in the DES arm and afterwards according to physician criteria. Statins were given to all patients indefinitely.
Study endpoints
The primary endpoint of the study was to compare overall costs (included in-hospital and follow-up) of both revascularisation strategies at one, two, three and five years of follow-up. Costs (expressed in US dollars; $) included hospitalisation, medications (procedural and follow-up) and procedural resources (initial and follow-up). Professional fees during PCI procedures were estimated according to national fees. Costs were determined from the perspective of third party payers based on the Argentina medical tariff in 2005-2006, and reflect procedures cost, stents used and days spent in hospital. Specific costing was done for each patient.
The same stent list prices were used in all patients (mean price of rapamycin- eluting stent $1991, paclitaxel-eluting stent $1924, zotarolimus-eluting stent $1827and BMS $493). The cost of oral sirolimus in Argentina for 14 days of treatment was estimated as $645. One month treatment with clopidogrel (Plavix, Sanofi-Aventis, Paris, France) was projected to be $50.60.
Secondary endpoints included safety endpoints defined by the incidence of major adverse cardiovascular events (MACCE), which included death from any cause, ST and non ST elevation MI and stroke.
Target vessel and target lesion revascularisation (TVR and TLR respectively) were analysed separately as efficacy endpoints and were not included as part of the MACCE definition. In the DES group, if repeat revascularisation was performed due to restenosis of a BMS, the event was classified as a repeat PCI (including costs), but not counted as TLR or TVR. All endpoints were analysed by the intention-to-treat principle. Stent thrombosis was defined as confirmed stent thrombosis when the patient had angiographically documented stent thrombosis with TIMI flow 0, 1 or the presence of flow-limiting thrombus. Suspected, stent thrombosis, was also recorded, and included cases where a patient suffered an acute ST elevation myocardial infarction corresponding to the DES target vessel, or suffered unexplained sudden death. This definition was previously reported10 and is consistent with the category of definite, probable or possible stent thrombosis as recommended by the Academic Research Consortium8.The diagnosis of acute MI was based on typical chest pain combined with either new pathological Q waves or an increase of creatine kinase to more than three times the upper limit of normal, with a concomitant increase in the MB isoenzyme. Sirolimus compliance and adverse side effects related to oral administration of sirolimus were also recorded. Follow-up coronary angiogram was performed if clinically indicated. All patients were scheduled for thallium stress testing between six to nine months after PCI.
Statistical analysis
The sample size of the study was determined on the basis of a test for a trend analysis. Based on our previous data with oral sirolimus and DES, we predicted that the incidence of TVR would be similar with both revascularisation strategies; in our previous studies with oral sirolimus19, the TVR rate of 8.3% at one year was similar to the rate of 8.8% reported by the ERACI III registry with DES20 and other recently randomised data with DES21.
In determining the power to detect a significant difference in the primary endpoint of overall cost between treatment groups at one year, a two-sided test for differences in independent binomial proportions with an alpha error of 0.05 was used. We calculated that approximately 100 patients needed to be treated to provide adequate numbers of patients with similar demographic, clinical, angiographic baseline characteristics in both groups and guarantee a power of 90%. Taking into account that both procedures share indirect costs, we only analysed direct costs and cost differences among both revascularisation strategies. A one-tailed, non-inferiority test was performed using a predetermined non-inferiority threshold level, with an overall alpha equal to 0.05, and was estimated using different follow-up and mean follow-up times. The hypothesis was that if the average DES cost minus the average of OR plus BMS cost was greater than the pre-specified non-inferiority threshold level, then DES would not be cost effective or cost saving compared to the OR group22. A bootstrap method was also used to validated the non-inferiority cost test. Cost analysis was performed by the economic department of the Argentina Ministry of Heath.
Continuous variables were expressed as mean±SD and categorical variables as percentages. Continuous variables were compared using ANOVA with Bonferroni correction. Categorical variables were compared using chi square analysis or Fisher’s exact test. Freedom from adverse endpoints at follow-up were obtained using Kaplan Meier curves and compared by the log rank test.
Univariate and multivariate Cox regression analysis was performed using SPSS v14.0 (intro mode: all variables introduced in block in a single step) to determine independent predictors of poor outcome at follow-up. Variables of statistical significance after univariate analysis and clinically relevant covariates including all demographic, clinical, angiographic and procedural variables were included in the analysis.
A p-value of <0.05 was considered statistically significant.
Results
Between January 2006 and September 30, 2007, 1274 patients underwent coronary angiography at the catheterisation laboratories of three centres in Buenos Aires, Argentina (Sanatorio Otamendi, Las Lomas and Clinica IMA). Those patients, who met the clinical and angiographic indication for inclusion, consent with the study design and were able for DES deployment, were randomised (200 patients, 15.6% of the entire population, 1). One hundred patients were included in OR group (131 vessels and 158 lesions) and 100 patients in DES group (142 vessels and 170 lesions). A total of 347 stents were deployed, 171 in the OR and 176 in the DES group. Baseline demographic, clinical, angiographic characteristics and procedural variables among both groups are described in Table 1. In the DES group, lesions were treated with Taxus stent in 87, Endeavor in 52, Cypher in 9 and Eucatax (paclitaxel-eluting stent with biodegradable polymer) in five lesions. Procedural and hospital success were similar in both groups (Table 2). Four patients in the OR group and one in DES group suffered a non ST elevation MI during initial hospitalisation. One additional patient in the DES group suffered an acute side branch dissection after stent deployment and needed an emergency PCI and stent deployment. This patient developed an ST elevation MI during hospitalisation. Seven days after hospital discharge, one additional patient in the DES group had a subacute stent thrombosis with an anterior ST elevation MI. During the first 30 days after PCI, in the DES group, two patients died, one due to a stroke and the other suddenly 28 days after PCI.
During the course of treatment with oral rapamycin, 24% of patients had minor or modest side effects after hospital discharge but only four (4%) discontinued the treatment. Complete blood count are seen in Table 3; white blood counts, although not significant, were reduced after rapamycin treatment (severe leucopenia was not seen) and the most frequent side effects were gum sores (14%) and diarrhoea (12%). None of these patients with undesirable side effects required hospitalisation and they had complete relief of the symptoms when the drug was stopped.
One year clinical follow-up
A mean of 18.3±7 months (range nine to 30 months) clinical follow-up was obtained in all patients in both groups (Table 2). During follow-up, there were seven deaths in the DES group (7%) and three (3%) in the OR group (p=0.36). Four deaths were defined as cardiac and three as non-cardiac. Three deaths occurred in the OR group, one was cardiac and the others two non-cardiac (Table 4).
After the first month, and until the end of the follow-up in the OR group, two MI were recorded, whereas, in the DES group, four patients had a myocardial infarction during follow-up, three were ST elevation MI. The follow-up incidence of clinical events are seen in Table 2.
The rates of the clinically driven TLR or TVR were similar with both strategies: TVR was 10.6% with OR versus 10.5% with DES and TLR per lesion was 7% with OR versus 8.2% with DES (Table 2 and Figure 2). TLR was performed for angiographic in-stent restenosis in all cases in the OR group, whereas TLR was performed for in-stent restenosis in nine patients and for stent thrombosis (confirmed or suspected) in the last three patients in the DES group. Thus, TLR for in-stent restenosis per lesion in DES group was 6.4 % (11/170). Repeat PCI in a non-target vessel was needed in three patients in the OR group vs. two patients in the DES group (p=ns). The number of the TLR rate was similar if we analysed only the cohort of 180 patients with one or more years of follow-up (11% in each group). The TLR rate according to DES design is described in Table 2.
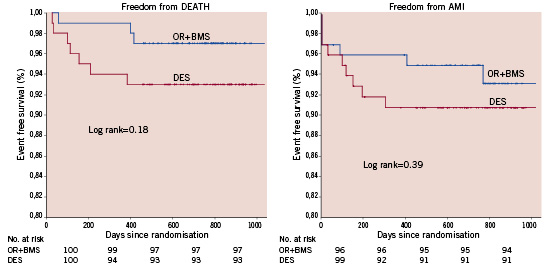
Figure 2. Kaplan Meier: freedom from target lesion (3A) and target vessel (3B) revascularisation.
Survival and survival-free from MI were similar in the two arms (Figure 3); patients initially treated with OR had a trend to improved freedom from MACCE compared to those treated with DES (91% vs. 85% respectively p=0.18) (Figure 4). During follow-up, 15% of the patients in each group had a recurrence of angina or developed an ischaemic thallium stress test, but at the end of follow-up, 92% of the OR and 91% of the DES patients were angina-free. Compared to baseline, the thallium stress test at six months showed normal or improved perfusion in 84% and 87% in OR and DES group respectively p=0.98. Baseline mean ejection fraction with scintigraphy was 56.3±11.8 in OR and 56.5±12.2.12 in DES groups, p=0.94.
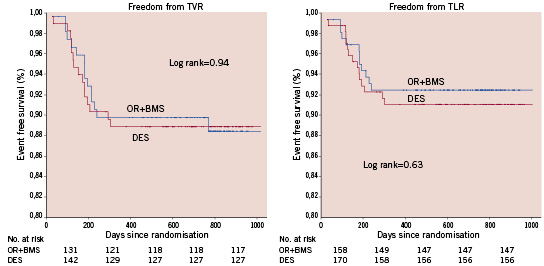
Figure 3. Kaplan Meier: freedom from death and myocardial infarction (ST and non ST elevation).
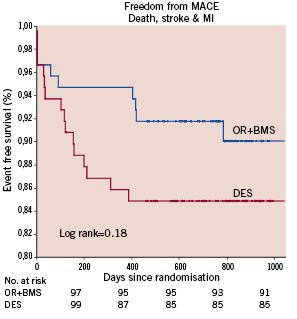
Figure 4. Kaplan Meier: freedom From MACCE (death, myocardial infarction and stroke) in both groups.
Procedural, hospital and overall costs
Table 5 summarises the hospital, follow-up and cumulative costs. Cost for the 14 days of oral sirolimus was added to the initial procedure cost in the OR group.
Follow-up costs included new revascularisation procedures, hospitalisation related to cardiac reasons and medications requirements of each arm. During follow-up, subsequent costs incurred differ significantly between groups. This reflects the fact that similar numbers of patients in either group required repeat revascularisation, but patients in the DES group required clopidogrel during the entire follow-up period. In contrast, patients in the OR groups received dual antiplatelet therapy for one month and thereafter aspirin alone.
At the end of follow-up, patients in the OR group sustained a significant lower cumulative overall cost than those treated with DES.
Non-inferiority testing determined that DES therapy failed to be cost effective with OR in all possible cost scenarios (hospital, follow-up and overall). Thus, for DES strategy to be cost effective relative to an OR plus BMS strategy, the procedural, follow-up and overall cost would need to be reduced by 17.4%, 18.5% and 20.2% respectively. In effect, cost differences between treatment strategies largely reflected the initial differences between the cost of DES and BMS. These differences in favour of BMS and OR, remained significant during and after the first year of follow-up due the similar incidences of TVR and TLR in the two groups (Table 2 and 5). Lastly, if we factor in the costs of patients with new adverse cardiac events, and generate 100 samples costs of patients with reposition through the bootstrap method, calculating for each sample the total amount of patients with complications and average costs, we collect the data represented in Figure 5. This graph shows that on the basis of the original sample and taking many random data, DES costs surpass widely those of BMS+OR and with greater dispersion.
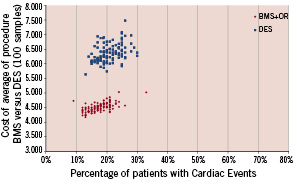
Figure 5. Bootstrap analysis in patients with adverse cardiac events in both groups.
Multivariate predictors of outcome
Multivariate logistic regression analysis of the study groups did not identify any independent baseline demographic, clinical or angiographic predictors of adverse outcome. The variables analysed into the model were sex, age, hypertension, diabetes, high cholesterol, renal failure, smokers, angina status, unstable angina, previous AMI, previous PCI, previous stroke, low ejection fraction, multivessel disease, left anterior descending artery disease, left main stenosis, number of treated vessels, number of treated lesions, number of stents, stent length, overlapping stents and treatment assignment.
Reference vessels less than 2.5 mm were the only independent predictor that trended towards a higher incidence of MACCE (OR: 0.42; CI:0.18-1.01 p=0.053). In contrast, univariate and multivariate analysis of predictors of TVR did not reveal any independent variables associated with poor outcome.
Discussion
This prospective, randomised controlled clinical trial demonstrates that an initial strategy with oral rapamycin plus BMS is a cost saving alternative compared to DES at 18 months of follow-up for the treatment of patients with de novo coronary lesions, with comparable efficacy, but significantly lower overall costs.
Furthermore, the sample population analysed in the present study represents a relative real world population, as reflected by the presence of diabetes in 28.5%, vessel size <2.5 mm in 32%, overlapped stents in 26% etc. Recently, several of this subsets of lesions met the off-label indication for DES, which has been associated with poor outcome23,24.
Since the introduction of DES into clinical practice, we have learned that DES is associated with a significant reduction of late loss compared to BMS. These values1-5,8,25-26 are lower than those obtain with systemic immunosuppressive drugs17,18, indicating that local therapy with DES is highly effective. All these findings suggest that the major benefit derived from DES is the suppression of neointimal hyperplasia, which is sustained at long-term follow-up. In agreement with this, our overall TLR rate in the DES arm was 8.2%, similar to 8.9% previously reported in the ERACI III using SES and PES with similar patient/stent ratio20. Additionally, our TLR rate in the DES group for lesions presenting with in stent restenosis was only 6.4% after 18 months of follow-up. However, it is not clear how this reduction in an angiographic endpoint such as late loss translates into better clinical outcome27-30.
When we considered the cost effectiveness comparison between DES and BMS, the final cost saving analysis was dependent upon the differences in the rate of repeat revascularisation procedures between BMS and DES during the follow-up, which is routinely high in the BMS group31-33. That was not the case in the ORAR III study and subsequently our non-inferiority cost analysis demonstrated that a significant reduction in the initial DES price would be necessary to be cost saving and effective compared with OR during the entire follow-up (including hospital and follow-up costs). However, lower prices of drug-eluting stents alone are unlikely to result in such stents being cost effective in all patients, until the problem of late stent thrombosis is solved with new generations of drug-eluting stents. Furthermore, in the present analysis, we had an unjustifiably high cost of oral sirolimus in Argentina, implying that this strategy could be even more cost saving if a lower price for the oral therapy could be applied. Additionally, the prices of bare-metal stents have decreased in the last two years, and these reductions may lead to a somewhat larger price difference between the two stent types. Finally, the bootstrap method applied by us, validated the results of our non-inferiority cost analysis.
Long-term dual antiplatelet regimen of aspirin and clopidogrel is the standard treatment for the prevention of late and very late stent thrombosis associated with DES34-36. Retrospective studies have shown that discontinuation of clopidogrel, even after six months or later following stent implantation, is related with an increased risk of thrombotic events in patients with drug-eluting stents7,11,37-39. Also, recently it has been reported that an incomplete response to clopidogrel was an independent predictor of stent thrombosis in patients receiving DES39. Although, we do not routinely assess platelet reactivity in our patients, it is clear that in those unable for long-term antiplatelet therapy or non-responsive to clopidogrel, alternative revascularisation strategies, such as the one presented here, have the potential to reduce the risk of thrombotic events.
Lastly, although almost 25% of patients developed minor or mild side effects related to oral rapamycin therapy, symptoms were relieved entirely after discontinuation of the drug.
In conclusion, the use of oral sirolimus in the prevention of coronary restenosis would have advantages and disadvantages compared to local infusion of the drug with DES implantation. Concerning the advantages, we should count that only minor and transient systemic side effects were present with the oral therapy; also, a BMS was implanted and therefore, safety concerns more frequently associated with DES as late incomplete stent malapposition, lack of neointimal coverage, inflammation etc.6,12,34-36 would be less frequent with this approach; and finally, in patients with multiple stent implantation, we can speculate there would be a greater cost saving effect. Regarding disadvantages, we should count the fact that oral therapy has a significant less pronounced neointimal inhibition compared to DES1-5,19, also, that oral therapy was given before PCI procedure and therefore its role during emergent procedures was not established for this experience; and finally, 4% of patients do not tolerate the drug and discontinue the treatment, a finding which was reproduced in all ORAR studies15,18-19.
Some limitations of the present study deserve comment. The open-label design of the study may have introduced the potential for bias. However, all adverse events were adjudicated by an independent committee that was unaware of the treatment group assignments of patients and patient care was clinically driven. Furthermore, the presence of side effects associated with the oral therapy15,17-19 compromise attempts to achieve a truly placebo controlled comparison. Secondly, even though the study size was appropriately powered for the primary endpoint, the sample size is not large. Also, we are presenting a relative short time of follow-up. We do not know if both strategies will become cost equivalent with a longer follow-up period. Also, although multiple DES designs with different amounts of late loss were used, non-clear superiority in terms of clinical outcome has been demonstrated with any specific head-to-head DES comparison, particularly when angiographic follow-up is not performed routinely40. In agreement with this, Endeavor III and IV trials, although they show significant greater reduction of late loss with SES and PES compared to ZES, long-term follow-up incidence of clinically driven TLR, target vessel failure and MACCE were similar. Furthermore, in Endeavor IV at two years of follow-up incidence of non Q MI was lower in ZES arm29,41. However, due small numbers of SES used in this study, any comparison can be draw with this stent design. Thirdly, the health care system in Argentina differs significantly from that of the US and European states and this may be perceived as a disadvantage of our analysis. We would argue however that the economic imperative to identify the most cost effective therapy for patients is universal, and this study provides impetus to replicate the results in other health care systems. Finally, only 15.6% of the total cohort of patients was included in the study, although this selection bias was similar to other randomised trials.
In conclusion, this randomised study with oral rapamycin in patients with de novo lesions, treated with coronary BMS implantation, demonstrates a significant cost saving with this regimen. This strategy may be a cost saving and effective alternative to DES therapy in a wide spectrum of patients undergoing PCI for de novo coronary lesions. However, a large scale, randomised study is required to confirm these findings.
ClinicalTrials.gov Identifier
NCT00552669
Appendix
The following participated in the ORAR III study:
Steering and Executive Committee:
Alfredo E. Rodriguez, MD; PhD, Principal Investigator (Argentina); Igor F. Palacios, MD (USA); Patrick W. Serruys, MD, PhD (The Netherlands); Andrew Maree, MD (USA); Sonia Tarragona PhD (Argentina)
Safety and Ethics Committee:
Jorge Tronge, MD (Buenos Aires, Argentina); Arnoldo Dubin, MD, PhD (Buenos Aires, Argentina); Cristina Sivori, PhD (Buenos Aires, Argentina); Alejandro Crespo PhD (Houston, USA); David Antoniucci, MD (Florence, Italy)
Clinical Events Committee:
Omar Santaera, MD (Chairman); Alberto Sampaolesi, MD (on behalf of Argentina Society for Cardiovascular Interventions); Miguel Russo-Felssen, MD; Valeria Curotto, MD
Coordinating Centre: Centro de Estudios en Cardiologia Intervencionista (CECI) Alfredo M. Rodriguez-Granillo, BSc
Economic analysis: Economic Department Argentina Ministry of Health, Sonia Tarragona, PhD
Statistics: Gaston A. Rodriguez-Granillo, MD, PhD; Sonia Tarragona, PhD
Cardiovascular imaging and scintigraphy: Gaston A. Rodriguez-Granillo, MD, PhD; Miguel Rosales, MD
Participating hospitals and clinical investigators:
Otamendi Hospital (Buenos Aires): Alfredo E Rodriguez, MD, PhD; Carlos Fernandez-Pereira, MD, Juan Mieres, MD, Gaston A. Rodriguez-Granillo, MD, PhD; Gustavo Risau, MD; Sanatorio Las Lomas (San Isidro-Buenos Aires) Juan Mieres, MD; Pablo Boskis, MD; Mario Boskis, MD
Clinica Medica Adrogue (Adrogue-Buenos Aires): Carlos Fernandez- Pereira, MD; Carlos Mauvecin, MD; Gustavo Allende, MD

