- budget-impact
- coronary stenting
- reimbursement
Abstract
Aim: To use an indirect comparisons approach and conduct a cost analysis comparing four drug-eluting stents (DES) from a United States (US) payer (i.e., fixed-fee reimbursement) perspective.
Methods and results: Studies were chosen that randomised two or more DES in diabetic patients. A one-year target lesion revascularisation (TLR) risk for Taxus was first derived. Risk Ratios (RRs) for each DES versus Taxus were calculated through meta-analyses. The RRs were multiplied by the average TLR risk for Taxus to estimate DES TLR risks. Estimates were added to a budget-impact model, along with utilisation and reimbursement rates for diagnosis-related groups. Budgets were calculated, assuming 100% stent use and 200,000 diabetic beneficiaries. One-year TLR risks were estimated to be 3.2%, 7.1%, 6.9% and 7.9% for Cypher, Endeavor, Taxus and Xience respectively. By substituting Cypher for DES with higher TLR, results predicted annual cost-savings greater than $146 million per population ($ 733 per patient). Results were comparable when assuming no difference in TLR risk between Endeavor, Taxus and Xience.
Conclusions: When outcomes from trials of diabetic populations are analysed and used in a budget-impact model from a US payer perspective, the use of Cypher is associated with lower TLR rates, which translates into large potential cost savings.
Introduction
The economic burden associated with coronary artery disease (CAD) is substantial, with costs estimated at $165 billion in the United States (US), and €45 billion ۠in the European Union1,2. Interventional techniques, such as use of percutaneous coronary intervention (PCI) with stenting are increasingly being employed as effective treatment options. However, restenosis and repeat revascularisations were important shortcomings of PCI with bare metal stents (BMS), costing the US Medicare programme more than $700 million annually3. The use of drug-eluting stents (DES) over the past decade has dramatically lowered restenosis rates, initially alleviating some of the associated clinical and financial burden1.
Health economic research for stenting in CAD has involved comparing DES versus BMS across regions globally through cost-effectiveness, database or budget-impact analyses3-9. A recent US analysis concluded that replacing BMS with DES increased total costs to the Medicare programme by $544 million because improved outcomes have encouraged wider use of PCI5. Currently, over 70% of PCIs are completed with DES in the US10 and commercially available stents include sirolimus-eluting stents (SES), paclitaxel-eluting stents (PES), everolimus-eluting stents (EES), and zotarolimus-eluting stents (ZES). With this rapid evolution in stenting, and increasing demands to limit the growth of health care budgets worldwide, a comparison of the health economic impact among DES is warranted. Only until recently have economic analyses been published that compare one DES versus another11,12, however, they do not compare all relevant DES.
Assessment of the comparative value of DES is best informed by head-to-head trials; however, such data are not always available. In the context of reimbursement decisions, this limitation is not new and alternative methods are often used13,14. Organisations that support reimbursement decision-making, such as the National Institute of Clinical Excellence (NICE), recommend that “if data from head-to-head randomised controlled trials are not available, indirect comparison methods should be used”14.
Using an indirect comparison framework, we conducted a one-year cost analysis comparing four major DES available in the US. A payer perspective, assuming fixed-fee reimbursement (e.g., Medicare) was used. The focus of this analysis was diabetic patients, as this population is at increased risk of restenosis compared to non-diabetics15,16. Although DES sold in the US have not received an FDA-approved indication for diabetes, these patients represent a high proportion (i.e., ~30%) of the PCI population receiving DES10.
Methods
A cost analysis was completed comparing SES (i.e., Cypher®; Cordis Corporation, Miami Lakes, FL, USA), ZES (i.e., Endeavor®; Medtronic Cardiovascular, Santa Rosa, CA, USA), PES (i.e., Taxus® Express2™ and Taxus® Liberte®; Boston Scientific, Natick, MA, USA) and EES (i.e., Xience™ V; Abbott Vascular, Santa Clara, CA, USA), hereon referred to as Cypher, Endeavor, Taxus and Xience. The analysis was conducted for diabetic patients receiving PCI with a DES who are reimbursed through a fixed-fee reimbursement system (e.g., Medicare). The model population size was estimated at 200,000 based on reported annual utilisation of inpatient PCI for the US (i.e., 1.3 million), a large US observational study of PCI patients (i.e., 70% have PCI with DES; 30% are diabetic), and an estimation of the population falling under fixed-fee reimbursement (i.e., 75%)1,10,17.
The one-year risk of target lesion revascularisation (TLR) was estimated for each DES by adjusting a weighted baseline Taxus risk of TLR by the risk ratios (RRs), which was obtained through pair-wise meta-analyses of Taxus versus DES. These estimates were used, along with costs associated with procedures, in a budget-impact model to predict one-year costs when assuming 100% utilisation of each stent in the market.
Indirect treatment comparison
An established indirect comparison methodology was used to inform clinical inputs13,14. This methodology involved three steps: 1) calculating a one-year weighted average risk of TLR for Taxus in diabetics based on findings of randomised studies; 2) conducting pair-wise meta-analyses to obtain a RR for TLR for each DES relative to Taxus; and 3) estimating absolute one-year TLR risk for each DES by multiplying the RRs from the meta-analyses by the weighted average Taxus TLR risk. This latter step informed the clinical inputs for the model.
The literature (PubMed) was searched from January 2005-May 2010 for randomised trials comparing a DES (i.e., Cypher, Endeavor, Xience) versus Taxus in a diabetic population (i.e., trial was restricted to diabetic patients) or diabetic sub-population (i.e., trial included all-comers but reported analyses for diabetic patients) that reported findings for TLR to one year. Taxus was chosen as the common comparator as this was the only DES that had published data compared to all other DES in diabetics. Recent (2008 to May 2010) conference proceedings (i.e., EuroPCR, TCT, ACC) were also searched to obtain data for newer, less studied DES (i.e., Xience, Endeavor). In total, 12 trials including 4,853 diabetic patients that fulfilled inclusion criteria were identified. Seven trials included Cypher (n=1,025), 12 included Taxus (n=2,001), two included Endeavor (n=491), and four included Xience (n=1,336)18-29. Table 1 provides studies and sample sizes and Appendix 1 provides trial characteristics.
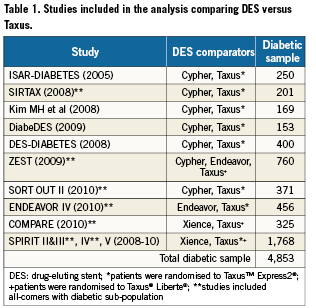
Meta-analyses of each DES versus Taxus were completed with Review Manager (Version 5.0; Copenhagen: Nordic Cochrane Center; 2008) using the “random effects” model. An adjusted indirect treatment comparison was also completed to determine whether statistically significant differences exist between Cypher vs Xience or Endeavor for annual TLR risk. The indirect comparison was performed using statistical software specific to conducting adjusted indirect comparisons30.
Cost parameters
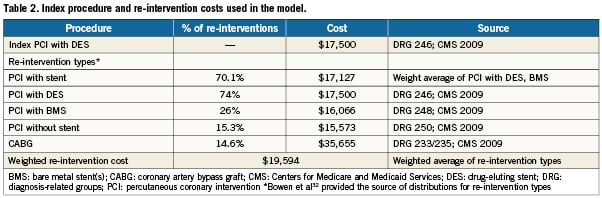
All costs used in the model were based on reimbursement rates for Medicare Severity Adjusted Diagnosis Related Groups (MS-DRGs)31. Specifically, the reimbursement rate for MS-DRG 246 (i.e., percutaneous cardiovascular procedure with DES) was used to estimate cost of index PCI with DES in diabetics, which were independent of DES type. This means that differences in DES price were not considered in this analysis. The re-intervention costs were a weighted average of all re-intervention procedure types. Since US prevalence could not be found, costs were weighted by prevalence of use reported in a Canadian observational study32 (Table 2). The reimbursement rate for MS-DRG 246 (percutaneous cardiovascular procedure with DES), MS-DRG 248 (percutaneous cardiovascular procedure with non-DES), MS-DRG 250 (percutaneous cardiovascular procedure without coronary artery stent), and MS-DRG 233/235 (coronary artery bypass with and without catheter) were used to represent reimbursement rates for different types of re-interventions. For coronary artery bypass, a 50/50 blend with and without cardiac catheterisation was assumed. It was also assumed that for diabetic patients, MS-DRG reimbursement rates specific to MCC (i.e., major complications and comorbidities) would apply (Table 2).
Cost analysis
For the analysis, a budget-impact model, capable of estimating the clinical and economic impact of using different DES, was used. For the model, one-year TLR risk and costs were estimated for each DES, assuming 100% utilisation of the stent for the estimated diabetic population undergoing PCI. In this analysis, Cypher had the lowest TLR rates among the DES analysed, and therefore, results are presented as the net budget impact (or cost savings) to the payer were Cypher to be used instead of each alternative DES.
The analysis was conducted using two methodologies for estimating TLR risk. The first method involved multiplying the exact point estimate RR (regardless of statistical significance), obtained from the meta-analyses, by the weighted average TLR risk for Taxus. The second method involved multiplying a RR value of 1.0, if meta-analyses showed non-significant results, by the weighted average TLR risk for Taxus.
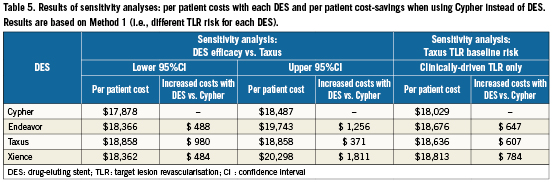
A sensitivity analysis was conducted whereby the upper and lower RR confidence intervals for the DES were multiplied by the weighted average TLR risk for Taxus. Another sensitivity analysis examined the cost impact of only including studies that measured clinically-driven TLR when calculating the baseline weighted average TLR risk for Taxus. Using these studies, a baseline risk of 5.8% was estimated for Taxus and relative treatment effects for each DES versus Taxus were applied to estimate DES specific TLR risk.
Results
Results of the pair-wise meta-analyses of DES versus Taxus are provided in Figure1. These results show that in diabetic patients, Cypher has a significantly lower TLR risk versus Taxus (RR=0.46; 95% confidence interval [CI]: 0.28 to 0.73; [p=0.001]) over one year. Both Endeavor and Xience show no significant differences in TLR risk versus Taxus, with RRs remaining close to 1.0 for both comparisons ([RR=1.03; 95%CI: 0.64 to 1.66; p=0.92 for Endeavor versus Taxus] and [RR=1.15; 95%CI: 0.64 to 2.07; p=0.64 for Xience versus Taxus]). There was no evidence of significant heterogeneity between trials within the meta-analyses (all p>0.10). The results of these meta-analyses, along with the weighted average TLR risk for Taxus of 6.9% (derived from randomised studies), were used to estimate one-year TLR risk for each DES (Table 3).
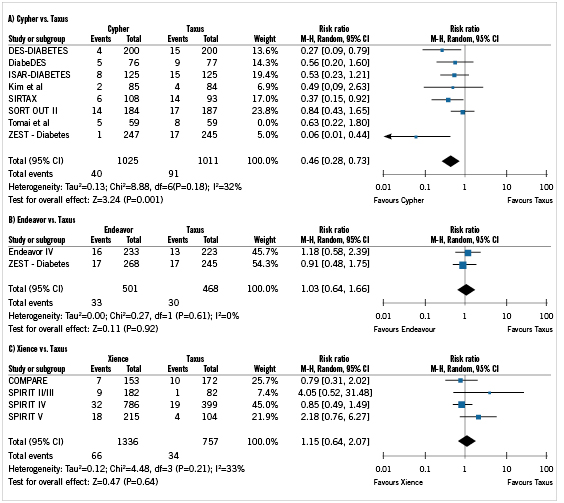
Figure 1. Forest plot of meta-analysis for one-year target lesion revascularisation in diabetic patients with Cypher versus Taxus (A), Endeavor versus Taxus (B), and Xience versus Taxus (C).
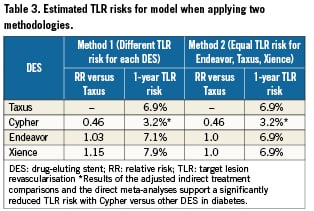
Results of the adjusted indirect treatment comparison supported the use of a different TLR risk for Cypher versus other DES in the cost analysis. Specifically, results showed that the difference in annual TLR risk was significantly lower for Cypher versus Endeavor (i.e., RR=0.45; 95%CI: 0.23 to 0.88) and for Cypher versus Xience (i.e., RR=0.40; 95%CI: 0.19 to 0.85).
Using the estimated TLR risks reported in Table 3 that were derived through our indirect treatment comparison, the results of our analysis predicted that annual costs of treating coronary artery disease with DES (i.e., including cost of stents, initial and repeat procedures) for an estimated 200,000 diabetic patients, is in excess of 3.6 billion dollars annually. These results were observed regardless of DES (Table 4).
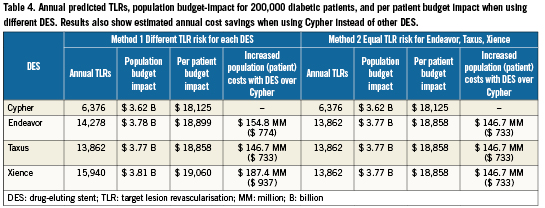
The model predicted that when substituting Cypher instead of DES with higher estimated TLR risk (Method 1), there would be annual cost savings ranging from $146 million to $187 million per population and from $733 to $937 per patient, depending on DES comparator (Table 4). Results also showed that use of Cypher still results in substantial cost savings versus use of Endeavor, Taxus or Xience under more conservative assumptions (Method 2). These analyses assume equal TLR risk for Taxus, Endeavor and Xience based on non-significant findings of meta-analyses.
Sensitivity analyses showed that substantial cost savings with Cypher were maintained (i.e., >$100 million regardless of comparator) when assuming alternative DES efficacy vs Taxus, as well as when only including studies that measured clinically-driven TLR (total budget impact not shown). Per patient cost savings ranged from $371 to $1,811 when using Cypher instead of other DES (Table 5).
Discussion
The availability of DES with proven efficacy in reducing restenosis means that decisions on adoption are either currently being made or need to be made by government, hospital and physician stakeholders. To facilitate such decisions, a global budget-impact model of DES was developed, capable of estimating the clinical and economic impact of using different DES for various populations. The present study provides an example of this adaptation according to a US payer (i.e., fixed-fee reimbursement) perspective for diabetic patients.
Results demonstrated that when outcomes from randomised trials of DES in diabetic patients are combined through indirect comparison, reductions in one-year TLR risk for Cypher translate into large potential cost savings for the US payer. Specifically, the model predicted that with 100% utilisation of Cypher instead of other DES with higher estimated TLR risk, there would be annual cost savings in excess of $146 million per population ($733 per patient). Regardless of the methodology used to estimate TLR risk for each DES, cost savings remained comparable, increasing the validity of these findings. The results are strengthened by the fact that estimates are informed from data representing a large diabetic sample of patients (i.e., n=4,853) and based on a well-established methodology used in health economics13,14.
To our knowledge, this study is the first cost analysis that compares the major DES used worldwide. There have been a few published analyses on use of DES in all-comers patients that focused on predicting budget impact with replacement of BMS with DES. For example, Greenberg et al33 used model estimations to conclude that use of DES (instead of BMS) in 80% of the PCI population was expected to increase annual US health care costs by ~$500 million. Ryan et al5 also found that introduction of DES in the US increased two-year costs to Medicare by $544 million. In the context of these studies, our results suggest that choosing a DES with lower re-intervention rates is one way to reduce the financial impact associated with increasing use of DES.
This study involved conducting several pair-wise meta-analyses between each DES and Taxus. Our results were corroborated by the findings of the most recently published study of Cypher versus Taxus in diabetics34 which reported an odds ratio of 0.41 (95% CI: 0.26 to 0.64) for TLR. Our results were comparable, specifically reporting a RR of 0.46 (95% CI: 0.28,0.73), which is not surprising considering that study inclusion was the same with the exception of the ZEST and SORT-OUT II results in the current analysis. Our study is also the first to report pooled results of the other DES versus Taxus in diabetics with reasonably large sample sizes of 969 patients for Endeavor versus Taxus and 2,093 patients for Xience versus Taxus. The results of the latter meta-analyses demonstrated no significant difference between either Endeavor or Xience versus Taxus.
The chosen methodology of conducting an indirect treatment comparison is well-established and continues to evolve13,14,35. The common issue necessitating an indirect comparison is that often a comparison between A and C is required but only direct comparisons between A to B and B to C exist. To provide guidance on fundamental principles of making indirect comparisons, organisations such as the International Society for Pharmacoeconomics and Outcomes Research, have developed “Indirect Treatment Comparisons Task Forces”36. They acknowledge that there are several techniques for indirect comparisons that can be applied. At the policy level, organisations such as NICE in the UK and Canadian Agency for Drugs and Technology in Health practice these methods.
Regardless of variation in methodologies and region-specific practices involving indirect treatment comparisons, one fundamental rule is that trial randomisation must be preserved; that is, a comparison of results from single treatment arms from different trials is not acceptable13,14. To abide by this rule, the methodology used in the current analysis makes indirect comparisons based on the common comparator of Taxus and preserves randomisation through use of relative risk treatment effects.
Most of the history of health economic research for coronary stenting has involved comparisons of DES with BMS through formal cost-effectiveness analysis (CEA) in North America, Australia, Asia and Europe4,6-9. Conclusions of such studies have generally depended on price differential between DES and BMS, with results showing better cost-effectiveness in populations at higher risk of restenosis. Given that there are limited economic comparisons of different DES, a logical extension of our study is to conduct CEAs comparing the major DES in different populations to address “value-for-money”.
The following study limitations are noted: 1) the Endeavor versus Taxus meta-analysis included the smallest sample of the meta-analyses. However, it must be noted that the diabetic subgroup of ENDEAVOR IV represents one of the largest direct comparisons of two commercially available DES in diabetic patients26; 2) the analysis focused on costs associated with procedures. Rare events such as stent thrombosis were not considered as larger sample sizes would have been required to make conclusions about differences. Past economic evaluations have sometimes included costs of these outcomes37,38; however, this may have been more relevant since comparisons were between DES and BMS; 3) costs associated with re-interventions were restricted to those required to complete it. The cost savings predictions may have thus been underestimated as other costs associated with angina symptoms (e.g., physician visits) that occur prior to, or in absence of a TLR were not considered39; 4)as we have only implemented one method for conducting indirect comparisons, it will be important to try to reproduce our results using additional methodologies, such as network meta-analysis; 5)finally, this analysis focused on FDA-approved DES. In Europe, there are newer DES available (e.g., Biomatrix), but an analysis could not be completed given insufficient data. However, some inferences can perhaps be drawn based on the European LEADERS trial which showed comparable clinical outcomes (i.e., MACE) in diabetic patients between Cypher and Biomatrix40). Such results would indicate a similar budget impact for each stent but this would need to be placed in context of DES price, if relevant.
Given that our analysis was completed from a US perspective, an understanding of the study generalisability to non-US regions such as Europe is worthy of discussion. First, both the indirect treatment comparisons methodology and results can be considered relevant from a European perspective. Such methodology is increasingly being used globally to inform decision-making as noted with NICE in the United Kingdom. As well, in the context of the DES market in Europe, such indirect comparisons are becoming increasingly necessary given the number of different DES approved for use. Further, clinical data used in health economic modelling studies can typically be generalised across regions if relative treatment effects are used, as was the case in our study41). This generalisation is also supported by the fact that the breadth of published literature available was considered, which included several European trials.
Second, as the clinical data can likely be generalised, similar overall economic conclusions may be expected despite variations in country-specific costs. In our study, the cost-savings results were driven by the relative clinical benefits of Cypher. Nevertheless, costing data (e.g., DES price if applicable) need to be locally adapted41. For instance, our study reflects a US payer perspective including a DRG-costing structure that does not require consideration of different DES prices. Such a cost would be relevant for country payers that reimburse according to DES type and price threshold analyses may be important to then consider. However, the types of resources and the costing analysis framework (e.g., quantification of the number of PCI’s), can be used as a starting point for local adaptations.
In conclusion, when outcomes from trials of diabetic populations are analysed and used in a budget-impact model based on a US payer perspective, the use of Cypher is associated with lower TLR rates which translates into large potential cost savings.
Conflict of interest statement
Ryan Saadi, Sidney Cohen and David Banko are employees of Cordis Corporation. Melissa Thompson, Michael Duong, Nicole Ferko are consultants for Cordis Corporation.

