Abstract
Aims: The TWENTE trial recently enrolled more than 80% of all eligible patients, who were randomised to zotarolimus-eluting Resolute or everolimus-eluting XIENCE V stents. In the present study, we investigated whether eligible, non-enrolled patients differed from the randomised TWENTE trial population in baseline characteristics and one-year outcome.
Methods and results: Characteristics of 1,709 eligible patients were analysed. Independent external adjudication of clinical events was likewise performed for non-enrolled (n=318) and randomised patients (n=1,391). Non-enrolled and randomised patients did not differ in gender distribution, diabetes mellitus, and clinical presentation, but differed significantly in age and cardiovascular history. Nevertheless, clinical outcome after one year did not differ in the primary composite endpoint target-vessel failure (TVF; 9.8% vs. 8.1%; p=0.34), and its components cardiac death (1.6% vs. 1.2%; p=0.61), target vessel-related myocardial infarction (4.7% vs. 4.6%; p=0.92), and target-vessel revascularisation (3.8% vs. 3.0%; p=0.48). Previous bypass surgery predicted TVF in non-enrolled patients (p=0.001); removal of these patients resulted in identical TVF rates for non-enrolled and randomised patients (7.3% vs. 7.3%; p=0.99).
Conclusions: Despite some differences in baseline characteristics, non-enrolled and randomised patients did not differ in one-year outcome, which was favourable for both populations and may be related to the drug-eluting stents used.
Abbreviations
CABG: coronary artery bypass grafting
DES: drug-eluting stent
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
TVF: target-vessel failure
TVR: target-vessel revascularisation
Non-STE-ACS: non-ST-elevation acute coronary syndromes
Introduction
Drug-eluting stents (DES) have been rapidly adapted for routine percutaneous coronary interventions (PCI), as they reduced the need for reinterventions.1,2 As first-generation DES did not improve mortality,3-6 novel stents with different coatings were developed, aimed at improved clinical outcome.7,8 Two of these so-called second-generation DES are the zotarolimus-eluting Resolute stent (Medtronic, Minneapolis, MN, USA) and the everolimus-eluting XIENCE V stent (Abbott Vascular, Santa Clara, CA, USA). Both DES have thin-strut, open-cell, cobalt-chromium-based stent platforms and thin, durable polymer-based coatings,9,10 and they have shown favourable clinical results that have led to widespread use in clinical practice.11-16 For these stents, non-inferiority with regard to safety and efficacy was recently demonstrated by TWENTE, a randomised, controlled study in a patient population with advanced coronary disease and complex lesions,17 which confirmed with relatively low event rates the results of the RESOLUTE All-Comers trial.18 In addition, TWENTE is one of the relatively few randomised comparative DES trials that have been performed in a study population with very limited exclusion criteria to reflect routine clinical practice.18-21
The enrolment in the randomised TWENTE trial was high, comprising more than 80% of all eligible patients.17 However, it is unknown whether the non-enrolled patients, who were all likewise treated with Resolute and XIENCE V stents, differ from the randomised TWENTE trial population in terms of baseline characteristics or –perhaps even more relevant– in clinical outcome. To answer this question, we prospectively recorded comprehensive data sets on clinical, procedural, and angiographic characteristics of all eligible but non-enrolled patients in the Non-Enrolled TWENTE study. To assure high-quality clinical outcome data and to facilitate meaningful comparisons with the findings of the randomised TWENTE trial, an external clinical research organisation performed the independent adjudication of all clinical events together in both the Non-Enrolled TWENTE study and randomised TWENTE trial.
Methods
STUDY DESIGN AND PATIENT POPULATIONS
Details of the randomised TWENTE trial, which was performed from June 18, 2008 to August 26, 2010 at Thoraxcentrum Twente in Enschede, The Netherlands, have previously been reported.17 TWENTE is a randomised, controlled, patient-blinded DES trial, comparing Resolute and XIENCE V stents after 1:1 randomisation (ClinicalTrials.gov NCT01066650). Patients were eligible for enrolment and randomisation if they were aged 18 years or older, were capable of providing informed consent, and underwent a PCI with DES implantation for the treatment of chronic stable coronary artery disease or non-ST-elevation acute coronary syndromes (non-STE-ACS). To include a broad study population, the study protocol defined no limit for lesion length, reference vessel size, and number of target lesions or vessels. The only exclusion criteria were: ST-elevation myocardial infarction (STEMI) or STEMI-equivalent requiring primary or rescue PCI during the past 48 hours; planned staged revascularisation; renal failure requiring haemodialysis; serious conditions that could limit the patient’s ability to participate in study procedures, in particular life expectancy <1 year; participation in investigational drug or device study; if the choice of stent type was dictated by logistic reasons (e.g., a stent with required dimensions only available as one type).17
During the course of the randomised TWENTE trial, patients who were not enrolled were also treated with one of both, Resolute or XIENCE V stents, and their clinical course was prospectively registered as part of the Non-Enrolled TWENTE study. Operators were asked to report reasons for non-enrolment in PCI reports but incomplete documentation of this detail was not infrequent. We therefore used PCI reports, all clinical records, and interviews with the operators and other medical staff involved to obtain the most reliable estimate of the reasons for non-enrolment. The Non-Enrolled TWENTE study and the previously reported randomised TWENTE trial complied with the Declaration of Helsinki for investigation in human beings, and were performed after approval and supervision of our institutional ethics committee.
INTERVENTION, MEDICATION, ELECTROCARDIOGRAPHY, AND LABORATORY TESTING
PCI procedures were performed according to standard techniques as previously described.17 In brief, lesion predilatation, direct stenting, and/or stent postdilatation were permitted at the operators’ discretion; liberal use of stent postdilatation was encouraged. Pharmacological therapy before, during, and after PCI as well as systematic laboratory and electrocardiographic testing were performed as previously described.17
DEFINITIONS OF CLINICAL ENDPOINTS
Definitions of clinical endpoints have been fully described in the main report on the randomised TWENTE trial.17 The same endpoint definitions were used in the present study. In general, the definitions of the Academic Research Consortium (ARC) were applied.22,23 In brief, the primary endpoint target-vessel failure (TVF) was defined as (in hierarchical order) cardiac death, target-vessel-related myocardial infarction, or clinically driven target-vessel revascularisation (TVR) by re-PCI or surgery. Cardiac death was defined as any death due to proximate cardiac cause, un-witnessed death and death of unknown cause, and all procedure-related deaths, including those related to concomitant treatment. Classification and location of myocardial infarction was performed based on laboratory testing, electrocardiographic parameters, angiographic information, and clinical data.17 Laboratory parameters for definition of myocardial infarction was any creatine kinase concentration of more than double the upper limit of normal with elevated values of a confirmatory cardiac biomarker.23 TVR was defined as any repeat coronary revascularisation of the target vessel. Target-vessel (or target-lesion) revascularisation was considered clinically indicated if the angiographic percent diameter stenosis of the then treated lesion was ≥50% in the presence of ischaemic signs or symptoms, or if the diameter stenosis was ≥70% irrespective of ischaemic signs or symptoms.22
Secondary clinical endpoints are: death from any cause; Q-wave and non-Q-wave myocardial infarction; any myocardial infarction; TVR by PCI, surgery, or either or both; clinically-indicated target-lesion revascularisation; any target-lesion revascularisation (stented segment including 5 mm proximal and distal border-zones); stent thrombosis, defined according to ARC.22 Composite parameters are (where applicable in a hierarchical order): Target-lesion failure, defined as a composite of cardiac death, target-vessel-related myocardial infarction, and clinically-indicated target-lesion revascularisation; and major adverse cardiac events, a composite of all-cause death, any myocardial infarction, emergent coronary artery bypass surgery or clinically indicated target lesion revascularisation.
DATA ACQUISITION AND FOLLOW-UP
In-hospital adverse events were recorded prior to discharge. As part of our centre’s standard follow-up procedure, 12-month follow-up data of all patients were obtained at visits to outpatient clinics or, if not feasible, by telephone follow-up and/or a medical questionnaire. For any event trigger, members of the study team gathered all clinical information available from referring cardiologist, general practitioner, and/or hospital involved.
INDEPENDENT CLINICAL EVENT ADJUDICATION
Processing of clinical data and adjudication of adverse clinical events of the Non-Enrolled TWENTE population were performed independently in the same way as for the randomised TWENTE trial (use of anonymous patient data and blinding for stent type) by Cardialysis in Rotterdam, The Netherlands. In brief, the clinical event committee adjudicated any death, potential myocardial infarction, stent thrombosis, and revascularisation.
STATISTICAL ANALYSIS
Data analysis was performed with the Statistical Package for Social Sciences (SPSS, version 17; SPSS Inc., Chicago, IL, USA). Data were reported as frequencies and percentages for dichotomous and categorical variables and as mean ± standard deviation for continue variables. The chi-square test and the Fisher’s exact test were used as appropriate. The student’s t-test was used to test normally distributed parameters. The Kaplan–Meier method was used to calculate the time to clinical endpoints and the Log-rank test was used to compare between-group differences. As non-enrolled patient populations are likely to contain more high-risk patients with a higher event rate,24 multiple logistic regression analysis was applied to the data of the non-enrolled patient population in order to identify predictors of TVF. In a subsequent analysis, we excluded patients with these variables to correct for potential confounders. Unless otherwise specified, a two-sided p value <0.05 was considered to indicate statistical significance.
Results
During the inclusion period of the randomised TWENTE trial, 2,239 patients were treated with DES at Thoraxcentrum Twente, The Netherlands. A total of 1,709 of these patients were eligible for study enrolment and randomisation. Finally, 1,391 of these 1,709 patients (81.4%) with 2,116 lesions were enrolled in the randomised TWENTE trial. In other words, only 318 eligible patients (18.6%, with 466 lesions) were not enrolled in the randomised trial but were assessed in the Non-Enrolled TWENTE study (Figure 1).
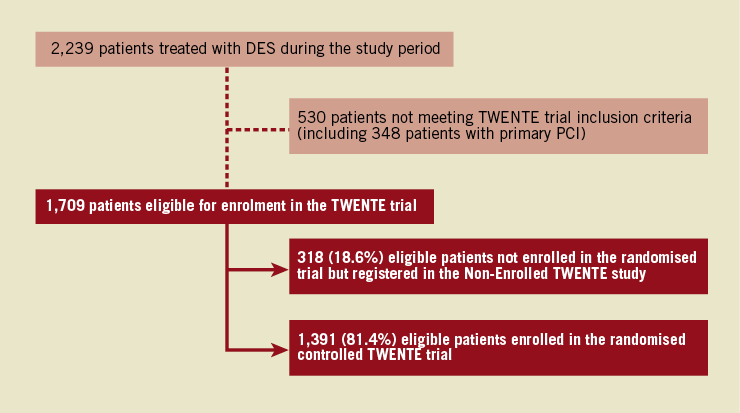
Figure 1. Flow chart of patients treated with DES during the course of the randomised TWENTE trial. Patients of the Non-Enrolled TWENTE study and the randomised TWENTE trial were compared. *Data of the randomised TWENTE trial have previously been reported.17
REASONS FOR NON-ENROLMENT
Reasons for non-enrolment and estimates of their incidence within the non-enrolled population were: (1) refusal of the patient to participate in the randomised trial (~10%); (2) uncertainty of the operator whether the information transfer was successful (e.g., because of language barrier, deafness, or the entire clinical condition) (~25%); (3) logistic reasons (e.g., an ACS patient is not informed prior to the catheterisation, while another patient is announced for primary PCI) (~15%); and (4) omission of informing the patient about the trial prior to an elective procedure (~30%). This means that a substantial proportion of the eligible patients (~20%; i.e., ~3.7% of all eligible patients) were not enrolled without evident reason.
PATIENTS, TARGET LESIONS, AND PCI PROCEDURES
Table 1 compares demographics and the procedural characteristics of both the Non-Enrolled TWENTE study population versus the randomised TWENTE trial population. Both study populations did not differ in the proportion of genders, diabetes mellitus, and clinical presentation (acute coronary syndromes in 52.5% vs. 51.5%, respectively; p=0.48). Non-enrolled patients were somewhat older (66.0±10.9 vs. 64.2±10.8 years; p=0.01). There was a trend towards less multivessel treatment in the non-enrolled patients (19.2% vs. 24.2%; p=0.06), matching with a more severely impaired left ventricular (6.5% vs. 3.0%; p=0.015) and renal function (6.6% vs. 2.7%; p=0.001) in this group. In addition, non-enrolled patients more often had a history of previous MI (43.1% vs. 32.4%; p<0.001), previous PCI (28.9% vs. 20.7%; p=0.001), and previous coronary artery bypass grafting (CABG) (17.0% vs. 10.6%; p=0.002; Table 1). A total of 466 and 2,116 lesions were treated in the Non-Enrolled TWENTE study and the randomised TWENTE trial, respectively (Table 2). Target lesions of non-enrolled patients more often showed complex B2 or C lesion types (76.1% vs. 70.1%; p=0.047). In parallel with the higher incidence of a history of PCI and/or CABG in the Non-Enrolled TWENTE population, more target lesions were restenoses and bypass graft lesions (p<0.001 for both; Table 2).
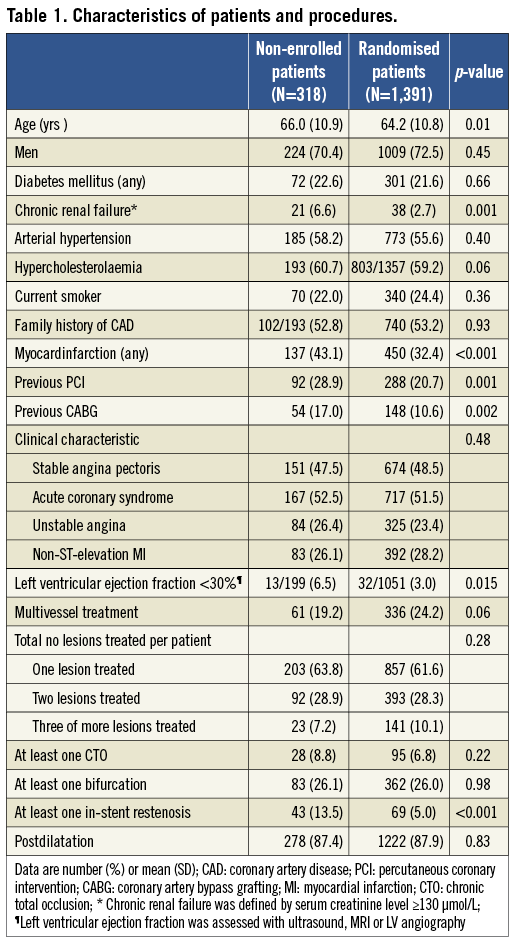
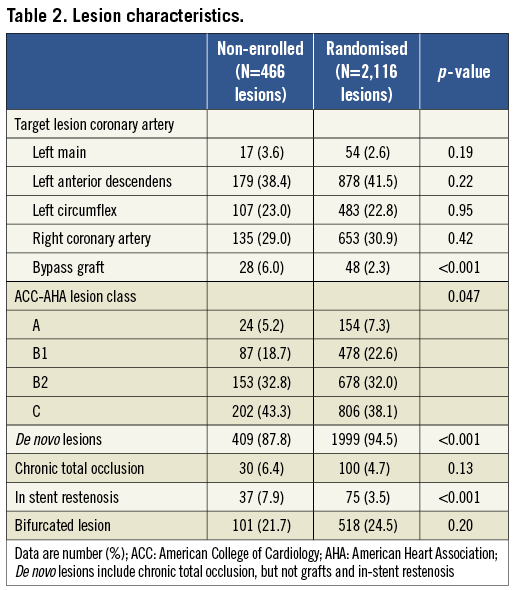
CLINICAL OUTCOME
Clinical follow-up data were available for 316 patients of the Non-Enrolled TWENTE study (99.4% follow-up data) and 1,387 randomised TWENTE patients (100% follow-up data available; four patients withdrew consent). Table 3 and Figure 2 show various clinical outcome parameters at one-year follow-up. Between both populations, there was no significant difference in the primary outcome parameter TVF (9.8% vs. 8.1%; p=0.34, OR 1.23 [95% CI 0.81 to 1.8]). There was also no significant difference in the components of the primary endpoint (cardiac death [1.6% vs. 1.2%; p=0.61]; target vessel-related MI [4.7% vs. 4.6%; p=0.92]; and clinically driven TVR [3.8% vs. 3.0%; p=0.48]), and any other clinical endpoint, such as death from any cause (2.2% vs. 2.1%; p=0.89) and major adverse cardiac events (9.5% vs. 9.5%; p=0.99; Table 3).
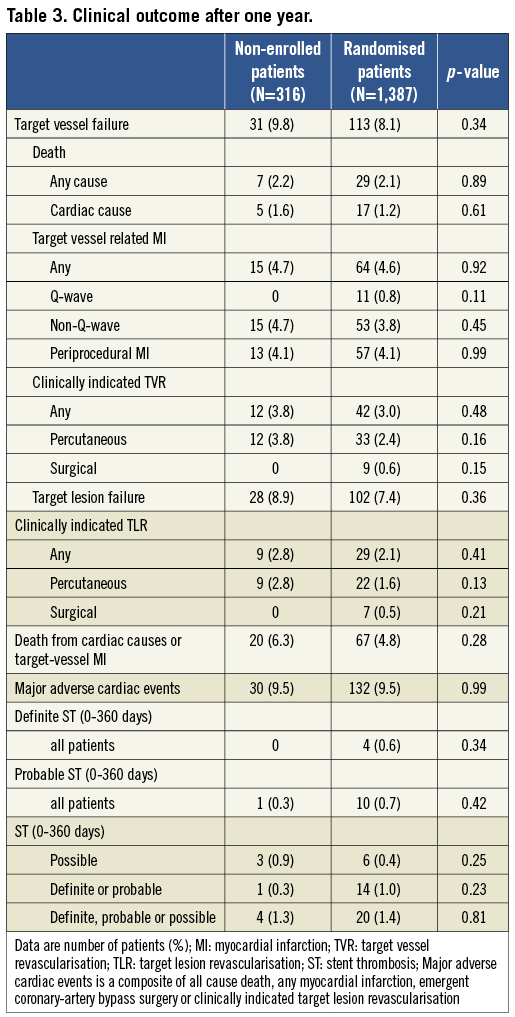
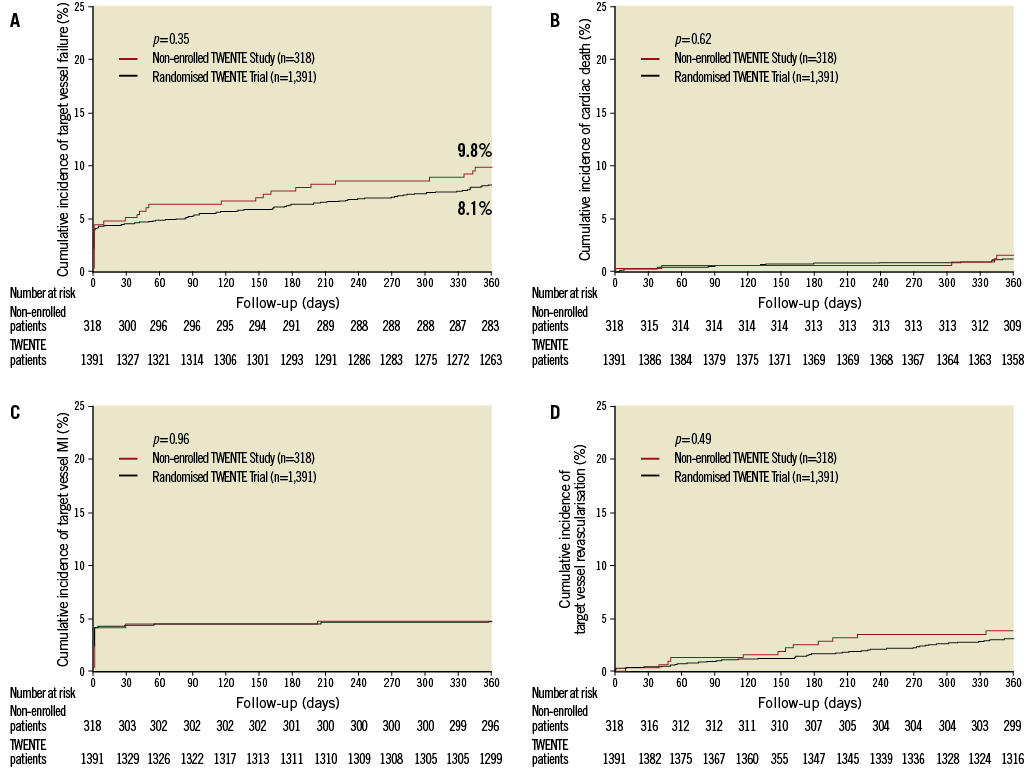
Figure 2. Kaplan-Meier for the primary endpoint and the individual components of the primary endpoint. Kaplan-Meier cumulative incidence curves at one year for the primary endpoint target-vessel failure, a composite of cardiac death, target-vessel related myocardial infarction, or target-vessel revascularisation (A); cardiac death (B); myocardial infarction (C); and target-vessel revascularisation (D) for both patients of the Non-Enrolled TWENTE study and of the randomised TWENTE trial.
STENT THROMBOSIS
Within the non-enrolled patient population, there was no definite stent thrombosis (Table 3). Definite or probable stent thrombosis occurred in one patient of the Non-Enrolled TWENTE population (one probable stent thrombosis) and in 14 patients of the randomised TWENTE trial population (0.3% vs. 1.0%; p=0.23).
PREDICTORS OF TARGET VESSEL FAILURE
The only parameter that significantly predicted TVF in the Non-Enrolled TWENTE population was a history of CABG (OR 3.7, 95% CI 1.67–8.15; p=0.001). After removal of patients with a history of CABG from the analyses (54/316 non-enrolled [17%] and 148/1,386 randomised patients [10.6%]), differences in baseline characteristics were virtually unchanged: the Non-Enrolled TWENTE population still comprised older patients (65.3±11.1 vs. 63.7±10.9 years; p=0.03) and more patients with severely impaired left ventricular function (6.2% vs. 2.6%; p=0.02), impaired renal function (5.3% vs. 2.6%; p=0.02), history of previous MI (42.8% vs. 31.5%; p<0.001), and history of previous PCI (24.6% vs. 18.8%; p=0.03). However, removal of patients with a history of CABG resulted in identical TVF rates for Non-Enrolled TWENTE patients and the randomised TWENTE population (7.3% [19/262] vs. 7.3% [90/1,239]; p=0.99). Moreover, the slight numerical differences in other clinical endpoints continued to be statistically non-significant (major adverse cardiac events 8.0% [21/262] vs. 8.6% [106/1,239]; p=0.78).
Discussion
In the present study, we addressed the question of whether patients, who were not enrolled in the randomised TWENTE trial17 but were all likewise treated with Resolute or XIENCE V stents, differed from the enrolled and randomised patients in baseline characteristics, procedural details, or clinical outcome. During the course of the randomised TWENTE trial, only 19 percent of the eligible patients were not enrolled in the randomised trial.17 To assure high-quality clinical outcome data and to facilitate meaningful comparisons, an independent external clinical research organisation performed the clinical event adjudication for both the Non-Enrolled TWENTE population and the randomised TWENTE population (together in the same adjudication session). The randomised TWENTE population comprised many complex patients and advanced coronary lesions,17 and in the Non-Enrolled TWENTE population many patients showed similar baseline characteristics and cardiovascular risk factors. Nevertheless, Non-Enrolled TWENTE patients were on average slightly older and more frequently showed a history of previous myocardial infarction and/or coronary revascularisations. As a consequence, we also identified mild but statistically significant differences in the rates of heart failure, renal failure, and lesion complexity in favour of the randomised TWENTE trial population, which comprised less bypass graft lesions and restenoses.
Despite the slight aforementioned baseline differences, the Non-Enrolled TWENTE population and the randomised TWENTE trial patients showed no significant difference in clinical outcome parameters such as TVF (9.8% vs. 8.1%; p=0.34), all-cause mortality (2.2% vs. 2.1%; p=0.89), or major adverse cardiac events (9.5% vs. 9.5%; p=0.99). Our data suggest that if all 1,709 consecutive eligible patients had entered the randomised trial, the overall TVF rate could have been as low as 8.5%. In fact, this study underlines the high clinical performance of the second-generation DES that were used. This performance appears to be greatly independent of the clinical profile of the patients.
COMPARISON WITH PREVIOUS STUDIES
Compared to RESOLUTE All-Comers trial18 and COMPARE trial,20 two randomised studies with second-generation DES in “real-world” patient populations, the randomised TWENTE patients showed similar or slightly higher rates of previous MI (32.4% vs. 16.5-29.7%), previous PCI (20.7% vs. 13.5-32%), previous CABG (10.6% vs. 6.5-9.8%), heart failure (3.0% vs. 2.5%), in-stent restenosis lesions (5.0% vs. 2.5-8.1%), bypass graft lesions (2.3% vs. 2.0-2.5%), and their age was similar (mean age 64.2 vs. 63.3-64.3 years). Accordingly, it is fair to state that the randomised TWENTE trial17 is a study in a “real-world” patient population (with the exception of acute STEMI), providing data that is highly relevant for routine clinical practice.
Analyses of randomised intervention studies that compared PCI and CABG have demonstrated that patient characteristics and the clinical outcome of these studies differed significantly from routine clinical practice.24 Selection bias is more likely to be undetectable in studies with low enrolment rates, but in the randomised TWENTE trial the enrolment rate was particularly high. In many Non-Enrolled TWENTE patients there was at least one reason for non-enrolment. Nevertheless, in approximately 3.7% of all eligible patients the main reason for non-enrolment could not be identified. This leaves room for potential selection bias, and in fact, the differences in baseline characteristics between the Non-Enrolled TWENTE study population and the randomised TWENTE trial patients suggest that there could have been some selection bias. Examples of patients whom operators may deliberately not enrol in a randomised trial are patients with target vessels that supply previously (partly) infarcted myocardium because persistent electrocardiographic changes may render the diagnosis of a subsequent myocardial infarction difficult and sometimes impossible. The same may apply to certain patients with previous CABG and end-stage coronary artery disease, who likewise often have a higher cardiovascular risk profile and an advanced age.
But what is known about eligible patients who were not enrolled in other randomised, comparative DES trials with “real-world” patient populations? In fact, such information is sparse. However, de Boer et al recently reported for their high-volume PCI centre baseline characteristics and one-year all-cause mortality of patients who participated in two randomised multicentre trials in all comers and compared it to non-participating PCI patients (579 patients enrolled vs. 663 non-participants).25 In that study, baseline characteristics differed significantly between trial participants and non-participants, who were older and had a higher incidence of heart failure and unstable clinical syndromes than trial participants).25 In addition, all-cause mortality at one-year follow-up was significantly higher in non-participants (6.9% vs. 3.1%; p=0.002).
Of note, these all-comers trials included patients with acute STEMI,18,19,25 which – on average – have a higher mortality risk. On the contrary, the randomised TWENTE trial did not enrol patients with acute STEMI,17 who consequently were also not assessed in the Non-Enrolled TWENTE study. In addition, de Boer et al addressed all non-participating PCI patients, including those who had clear contraindications for participation in one of the two randomised trials (e.g., patients in shock with very high mortality risk),25 while our own study examined only eligible patients who all fulfilled the inclusion criteria of the randomised TWENTE trial.17 This may explain differences in all-cause mortality between non-participants of the study of de Boer et al and the Non-Enrolled TWENTE population. A comparison of clinical outcome parameters other than mortality was not possible, as no such data were available for non-enrolled patients of other randomised comparative DES trials.
PREVIOUS BYPASS SURGERY AS PREDICTOR OF OUTCOME
In the Non-Enrolled TWENTE population, a history of CABG turned out to be the only predictor of TVF. In fact, the rate of TVF became identical for both patient populations after removing patients with a history of CABG from both patient populations (7.3% vs. 7.3%; p=0.99). Implication of this finding may be that particular attention should be paid to the distribution of patients with a history of CABG between the study arms of comparative DES trials.
Notably, in the randomised TWENTE trial17 the proportion of patients with a history of CABG was similar or even higher than in some recent trials with second-generation DES in all-comer populations.18,20
Study limitations
This trial was performed in a high-volume tertiary centre for PCI by five experienced operators with relatively uniform procedural strategies and liberal use of stent postdilatation.17 Therefore, generalisation of the results may be limited in other settings.
Conclusion
Despite some differences in baseline characteristics, non-enrolled and randomised patients did not differ in one-year clinical outcome, which was favourable for both populations and may be related to the second-generation drug-eluting stents used.
Funding
The Non-Enrolled TWENTE study is an investigator-initiated study that was performed without funding. The randomised TWENTE trial is an investigator-initiated study, supported by equal unrestricted grants from Abbott Vascular and Medtronic.
Conflict of interest statement
C. von Birgelen is consultant to and has received lecture fees or travel expenses from Abbott Vascular, Medtronic, and Boston Scientific; he received a lecture fee from MSD. All other authors have no conflict of interest to declare.

