Abstract
Aims: The present study was designed to evaluate a novel third generation bare-metal stent (BMS) comprised of an ultra-thin-strut, cobalt-chromium platform with fixed geometry, uniform cell size, and superior surface finish in a porcine coronary artery model.
Methods and results: A total of 47 BMS of two types were implanted in pig coronary arteries using QCA to optimise stent apposition: a commercially available cobalt alloy thin-strut stent (91µm) as control (Driver; n=17), and an ultra-thin-strut (65 µm) cobalt-chromium stent (Protea; n=18). Animals underwent angiographic restudy and termination one week and one month post-implant for coronary artery histology. In addition, 12 overlapping Protea stents were analysed at one month. At one week, comparable thin neointima and mild inflammation were observed in both groups. At one month, Protea demonstrated significantly lower angiographic % stenosis (2±1% vs. 17±5%, p=0.006), intimal thickness (0.11±0.01mm vs. 0.23±0.03 mm, p=0.003), and histologic % area stenosis (19±2% vs. 32±3%, p=0.003). Mean stent strut injury scores were low and similar between groups. Angiographic % stenosis, intimal thickness, and histologic % area stenosis of overlapping Protea stents were 3±1%, 0.13±0.01 mm, and 22±2%, respectively, and similar to the single Protea group. Stable fibrocellular neointimal incorporation, with complete endothelialisation and minimal inflammation, were observed at one month in all stents, including overlapped Protea segments.
Conclusions: When compared to a commercially available cobalt alloy BMS, the new third generation Protea stent demonstrated favourable coronary arterial response with significant reduction of neointimal formation in the porcine model. Our results showed how seemingly trivial improvements to the BMS technology can result in substantial biological responses. Future, long-term investigations are needed to ascertain the clinical applicability and implications of these findings.
Introduction
The innovation of intracoronary stents has resulted in significant reductions in acute vessel closure and restenosis in modern percutaneous coronary intervention. As a result, these devices have largely replaced stand-alone balloon angioplasty in most situations. Nonetheless, bare metal coronary stents (BMS) are still associated with angiographic and clinical in-stent restenosis rates of 20-30% and 10-15%, respectively.1-3
Subsequently, the pivotal Randomised Study with the Sirolimus-Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients with de novo Native Coronary Artery Lesions (RAVEL) and Sirolimus-Eluting Stent in De Novo Native Coronary Lesions (SIRIUS) trials evidenced dramatic target lesion revascularisation reductions for drug-eluting stents (DES), as compared to their bare metal predecessors.4,5 However, albeit rare, the potential risk for late DES thrombosis, with its attendant catastrophic sequelae, remains a concern.6-9 BMS thus remain the preferred platform in patients unable or unwilling to comply with prolonged dual antiplatelet therapy. Furthermore, if future BMS development results in enhanced restenosis advantages, it may offer an attractive alternative to DES.
Ongoing evaluations to improve currently available BMS have suggested an association between strut thickness and in-stent restenosis.10-12 Properties of the alloy cobalt-chromium allow for thinner stent struts. These devices demonstrate maintained radiopacity and improved deliverability, thus providing a potentially superior BMS, and possibly, DES platform.13,14
There is a renewed interest in BMS, and better BMS make better DES platforms. Improved stent architecture can potentially impact future benefits of not only BMS, but also DES. If such novel platforms enhance neointimal coverage, the risks of late DES stent thrombosis would be attenuated; conversely, if the design helped inhibit in-stent restenosis, the required drug dose may be decreased, thereby reducing vessel toxicity.
Our objective in this study was to evaluate a new third generation BMS characterised by an ultra-thin-strut, cobalt-chromium platform with fixed geometry, uniform cell size, and superior surface finish in a clinically relevant porcine coronary artery model.
Methods
Study design
Fourteen juvenile domestic pigs (30 to 50 kg) were enrolled in this study. Experimental procedures were performed in a standard fashion, without notable difficulties, according to the recommendations of the consensus advisory panel to the Food and Drug Administration15 and in compliance with the Association for Accreditation of Laboratory Animal Care. Stents were implanted according to a randomisation scheme with even distribution amongst the three epicardial arteries, constrained by individual anatomy, to reduce potential bias from specific arterial implant site responses.
A total of 47 stents of two different types were implanted in pig coronaries using quantitative coronary angiography (QCA) to optimise stent apposition: a commercially available cobalt alloy thin-strut stent with 91 µm strut thickness as control (Driver, Medtronic Corporation, Minneapolis, MN, USA; n=17), and an ultra-thin-strut (65 µm) cobalt-chromium stent (Protea; n=18). In addition, 12 overlapping Protea stents were deployed. All stents were 3.0 mm in diameter and 16 mm in length.
Animals, stent implant, terminal restudy, and sample preparation
Animals received 81 mg aspirin and 75 mg clopidogrel three days before stent implantation and daily until termination. Cardiac catheterisation was performed with full heparinisation (200 units/kg), maintaining an activated clotting time >300 s. Intracoronary nitroglycerin was given for vasodilatation during angiography, and stents were implanted using QCA to obtain stent: artery ratio≈1.15:1. Post-implant angiography and QCA were performed to determine stent size, complete apposition, and patency.
Four animals were terminated at one week post-implant (total of 12 stents, six stents per group), and eight animals at one month (23 stents: 12 Protea and 11 Driver) for coronary artery histology. In addition, two animals with total 12 overlapping Protea stents were sacrificed at one month. Stented coronary arteries were removed from perfusion fixed hearts, processed for histological analyses, and evaluated. At explant, coronary arteries were perfusion-rinsed with phosphate buffered saline and perfusion-fixed in situ at physiologic pressure with 5% formalin/1.25% glutaraldehyde for 15-20 min. Glutaraldehyde was added as a fast-acting, cross-linking fixative to better preserve the mechanical strength and stability of the native vessel structure.16 Stented vessels were trimmed free from the hearts and embedded in methyl methacrylate. Sections 5 µm thick from proximal, middle, and distal vessel regions were cut using a heavy-duty microtome, collected on glass slides, de-plasticised, and stained with haematoxylin-eosin and Verhoeff-Masson elastic-trichrome.
Stent design
The Protea was designed to be a superior BMS and better DES platform. The Protea stent is a third generation bare metal stent characterised by an ultra-thin-strut (65 microns), cobalt-chromium platform with fixed geometry, uniform cell size, and a unique superior surface finish when compared to currently available cobalt-chromium alloy stents (Figure 1). It is believed that the thinner the struts the less the injury and the faster the healing. The Protea has rectangular strut geometry and is a laser cut slotted tube. The stents sinusoidal rings all carry the same geometry, shape and size. This is to ensure equal distribution of drug along the stent when used as a DES platform. Previous prototypes of CoCr which we also tested had a variable geometry where the geometry, shape and size of the sinusoidal rings varied and hence had varying drug distribution of drug. Furthermore, regulatory purposes require equal distribution of drug along a stent (each ring should have the same amount of drug) and this can be reached only with uniform geometry. The cell size of Protea and the rectangular strut shape allows for less injury to the vessel wall due to intracellular balloon protrusion during stent deployment.
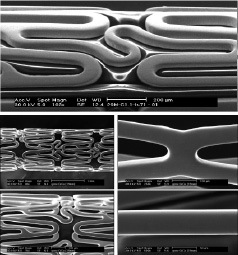
Figure 1. Protea ultra-thin-strut cobalt-chromium stent showing optimisation of cell size to reduce intracellular balloon protrusion and lower wall injury during deployment. The stent is 65 microns thin, much thinner than many of the other CoCr stents studied, and it expands in a uniform manner across its length, i.e., it does not «dog-bone» and may produces less injury during implantation. In addition, Protea stent features a unique surface treatment eliminating most of the intrusions inherent to the cobalt-chromium alloy.
Protea expands symmetrically whereby during stent expansion it directs the expansion forces at the balloon edges to the centre. This is expected to result in less injury at the edges. The rings that constitute the two ends of the stent are designed to eliminate dog-bone effect where the ends flare first before the rest of the stent expands. This is to reduce edge injury on deployment. Earlier CoCr stent designs feature a noticeable centre to edge expansion, while in the Protea it is less pronounced. The balloon is wrapped to eliminate balloon protrusion through the stent cells on inflation and eliminate additional injury; this is good for both BMS and DES. Balloon and stent are matched to reduce balloon overhang again to reduced inflation injury. These design features have obvious benefits for both BMS and DES.
The Protea CoCr platform also has an improved surface finish. The surface is ultra-smooth and processed to reduce tungsten carbide precipitates formed during processing of CoCr Alloy stents. In general, cobalt-chromium surface residue contains many precipitates from laser cutting which are not removed by ‘standard’ electro-polishing. Thus, the surface of a CoCr stent is not only rougher than stainless steel it also contains impurities. Tungsten oxide (also known as ‘white spots’) make up a large percentage of the surface precipitates. The goal was to get as clean a surface as possible for our BMS and future DES platform so the drug eluting coating has the best possible integration with the stent. We believe that this unique feature of ultra-smooth surface may improve the performance of the stent as a BMS and/or DES platform.
Histopathology
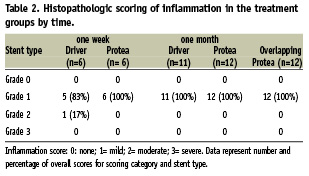
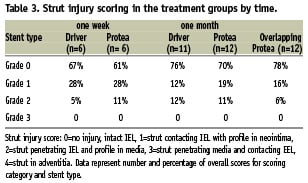
Proximal, middle, and distal sections from each stented segment were scored for inflammation and strut-associated injury. The nature of the extracellular matrix in the neointima and adventitia was assessed and described. An overall inflammation score was assigned according to the following semi-quantitative grading scale: 0=none; 1=mild, scattered inflammatory cells; 2=moderate, inflammatory cells encompassing <50% of a strut in at least 25% to 50% of the circumference of the artery; 3=severe, inflammatory cells surrounding a strut in at least 25% to 50% of the circumference of the artery.17 A modified scoring scale derived from the original injury score developed by Schwartz et al was used to grade the extent of vessel injury determined by stent strut penetration into the vessel wall as follows: 0=no injury, intact internal elastic lamina (IEL), 1=strut contacting IEL with profile in neointima, 2=strut penetrating IEL and profile in media, 3=strut penetrating media and contacting external elastic lamina (EEL), 4=strut in adventitia.18
Histomorphometry
Each vessel section was imaged by CCD camera, at an appropriate magnification. Morphometric analysis was performed by computerised planimetry using Image Pro Plus software (Media Cybernetics, Silver Spring, MD, USA) on proximal, mid, and distal sections of each stented vessel. The lumen, IEL, and EEL were traced; and area measurements were obtained. The areas of the neointima and media were obtained by subtraction of the lumen area from the IEL, and IEL from EEL, respectively. Neointimal thickness at each stent strut was measured directly (stent void not included). Histologic percent area stenosis was calculated using the following formula: [1-(luminal area/IEL area) x 100].
Statistical analysis
The authors had full access to the data and take full responsibility for the integrity of the data. Descriptive statistics were generated for all quantitative data and expressed as mean ±SEM. Angiographic and histomorphometric data were evaluated by one-way analysis of variance (ANOVA). All-pairwise multiple comparisons using the Tukey method were performed if ANOVA probability was <5%. For noncontinuous data (i.e., histopathologic scoring), Kruskal-Wallis one-way ANOVA on ranks was used.
Results
All stents were deployed successfully without notable performance difficulties. Angiographic, histopathologic, and histomorphometric measurements and analyses were completed for all stents. Table 1 illustrates the distribution of stents according to stent type and restudy time.
Angiographic follow-up
Baseline and post-deployment diameters of implant target sites were similar between groups. Stent-to-artery ratios were also similar: 1.19 ± 0.11, 1.12 ± 0.05, and 1.16 ± 0.02 for Driver, single Protea, and overlapping Protea, respectively (p=NS). Angiographic % diameter stenosis at one month was significantly lower for Protea compared to Driver (2±1% vs. 17±5%, respectively; p=0.006; Figure 2). There was no difference in% diameter stenosis between overlapping and single Protea stents (3±1% vs. 2±1%, respectively; p=0.661).
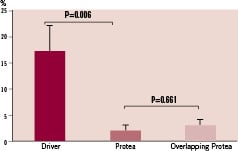
Figure 2. Angiographic % diameter stenosis at one month follow-up in stented pig coronary arteries.
Histopathology and histomorphometry: qualitative observations
At tissue harvest, gross examination of all hearts revealed no visible infarcts or other macroscopic abnormalities. Stents were consistently found well-embedded into the arterial wall structures, with compression injury to the tunica media and formation of a thin-to-moderate, generally concentric fibrocellular neointima with complete coverage of flattened periluminal cells.
At one week, thin neointima and mild inflammation were present and similar in both groups. The tunica media was compressed and thinned at the site of stent strut contact, and no medial haemorrhage or necrosis was observed. Inflammatory cell infiltrates were found at the luminal surface and in thin mural thrombi, as well as in the perivascular spaces and adventitia. These infiltrates, identified in both groups, consisted mainly of polymorphonuclear leukocytes (neutrophils) and monocyte-macrophages (Figures 3 and 4).
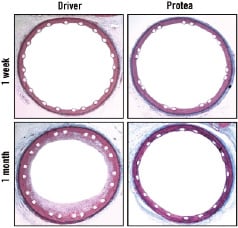
Figure 3. Low-magnification micrographs of pig coronary arteries harvested one week and one month after stent implant, demonstrating comparative overall vessel morphologies. There was mild compression injury to the tunica media and formation of a thin, mostly concentric fibrocellular neointima in response to both stents. However, at one month Protea showed attenuated neointima formation. Verheoff-Masson (VM) elastic-trichrome stain, magnification x20.
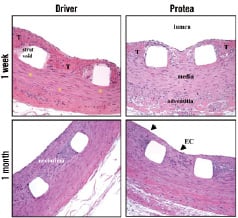
Figure 4. High magnification micrographs of pig coronary arteries harvested one week and one month after stent implant. Compression barotrauma of the medial tissue layers, without dissection injury, and minimal focal smooth muscle cell necrosis was observed in all groups (*). Thrombi (T) were present in the neointima for both treatment groups at one week, although to a greater extent in the Driver group. Furthermore, there was minimal inflammatory reaction throughout the vessel wall for all stent types; and a confluent layer of flattened endothelial cells was present at the luminal blood flow interface (EC - arrows). Protea showed attenuated intimal thickness at one month, compared to Driver. Hematoxyline & Eosin (H&E), magnification x200.
At one month, neointimal formation appeared thinner for the Protea group. Neointima was composed of proteoglycan-rich matrix in the deep regions and collagenous matrix in the more adluminal region. Moreover, there was a confluent squamous peri-luminal cell layer. Leukocyte involvement included moderate numbers of peri-strut multinucleated foreign-body giant cells as well as occasional diffuse or focal histiolymphocytic infiltrates with eosinophils in the adventitia and perivascular space. The adventitia demonstrated minimal reaction, mainly showing as fibrotic thickening. Overlapping Protea stent implants did not appear substantially different from single Protea stents. The vessels were well-healed with mild inflammation, and no luminal thrombus (Figure 5).
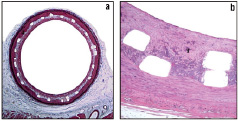
Figure 5. Low and high magnification microscopic images of sections from overlapping Protea samples harvested one month post-implant, a) Verhoeff-Masson (VM) elastic-trichrome stain, magnification x20, b) Hematoxyline & Eosin (H&E), magnification x200.
Intimal thickness
At one week, mean intimal thicknesses were similar between groups: 0.02±0.01 mm in the Driver group and 0.01±0.0 1mm in the Protea group (p=0.313). At one month follow up, intimal thickness was significantly lower for Protea compared to Driver (0.11±0.01 mm vs. 0.23±0.03 mm, respectively; p=0.003; Figure 6). Intimal thickness for the overlapping Protea was 0.13±0.01 mm, similar to single Protea stents (p=0.384).
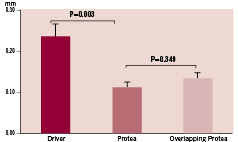
Figure 6. Intimal thickness of tissue overlying the stent struts, measured from histologic sections of porcine coronary arteries harvested one month after stent implant, according to treatment group.
Histological percent area stenosis
While no difference was noted in histological % area stenosis between Protea and Driver groups at one week (5 ± 1% vs. 8±1%, respectively; p=0.052), the value at one month was significantly lower for Protea group (19±2% vs. 32±3%, respectively, p=0.003; Figure 7). For overlapping Protea, histological % area stenosis was 22 ± 2%, similar to single Protea (p=0.349).
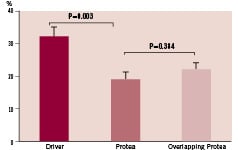
Figure 7. Proportion of area within stent occupied by neointimal tissue (% area stenosis) measured from histologic sections of porcine coronary arteries harvested one month after stent implant, according to treatment group.
Inflammation and strut injury scores
Mild inflammation was similarly observed in both groups at one week (p=0.074) and one month (p=1.000; Table 2). Stent strut injury score was low and similar between groups at one week (0.49±0.27 vs. 0.38±0.49, p=0.681) and one month (0.30±0.61 vs. 0.36±0.67, p=0.801; Table 3). Overlapping Protea segments demonstrated similar mild inflammation (p=0.480) and low strut injury scores (p=0.498), comparable to single Protea stents.
Discussion
This study aimed to assess the response of porcine coronary arteries to implantation of a novel third generation BMS, characterised by an ultra-thin-strut cobalt-chromium platform, with fixed geometry, uniform cell sizes, and superior surface finish. Stent geometric configuration and strut thickness have been demonstrated as important predictors of restenosis.10,11,19-21 In this regard, previous literature has reported that cobalt-chromium alloy, compared to stainless steel, has a higher radial strength and radiopacity with similar electro-negativity.22 These features allow for stent designs with thinner struts, thereby enhancing flexibility and ease of delivery, while maintaining radiopacity.13,23 Accordingly, we postulated that incorporation of fixed and uniform cell sizes, superb surface finish, with further reduction in strut thickness, could elicit favourable responses in the laboratory animal setting, providing a basis for clinical investigation.
Our data demonstrated favourable arterial response, with significant reduction in neointimal formation, associated with the study stent design, as compared to Driver, a widely used cobalt-chromium BMS,14,22-24 in porcine coronary arteries. In specific, angiographic % diameter stenosis, intimal thickness, and histological % area stenosis were all lower for the Protea versus the Driver stent. Moreover, overlapped deployment of Protea resulted in no changes in the above parameters. Thus, as a potentially superior stent platform design, Protea, in the absence of drug coating, appears to offer neointimal suppression advantage over control BMS. Such drug-associated heightened vascular toxicity in overlapped DES segments has been well documented in previous studies25. Follow-up values of intimal thickness, histological % area stenosis, inflammation score, and strut injury score for the control group (Driver) were in concordance with our earlier porcine data for cobalt-chromium stents,26 further substantiating the potential advantages of the study stent.
Neointimal hyperplasia is the major cause of in-stent restenosis. In general, neointima consists of a mixed population of inflammatory cells, myofibroblasts and smooth muscle cells that synthesise, secrete and deposit extracellular matrix components. In our study, morphological qualitative observations showed that neointima was thinner in the Protea implanted vessels compared to Driver group due to a lower content of the cellular component and less extra cellular matrix deposition in the peri-strut area and between the struts.
The biocompatibility and safety profile of the cobalt-chromium alloy has been evidenced by over a decade of incorporation into manufacturing of surgical implants.27 Furthermore, robust data substantiate its clinical safety and efficacy in the coronary circulation.13,14,22-24 In addition to enhanced radiographic visibility and flexibility, cobalt-chromium appears devoid of adverse proliferative responses that accompany implant of stents fashioned from other alloys (e.g., gold, martinsitic nitinol).28-30
Favourable biocompatibility profile is desirable not only for BMS, but also for potential consideration as an eventual DES platform.31,32 After drug elution, the residual polymer matrix and stent material become crucial determinants of late restenosis and device failure, as exemplified by the paclitaxel-eluting QuaDDS-QP2 device (Quanam Medical, Santa Clara, CA, USA).33
It has been postulated that, in animal models, stent surface material and geometric configuration may outweigh operator-dependent variables in eliciting neointimal hyperplasia and thrombosis.19 Thus, while reduced strut diameter is widely accepted as a deterrent of in-stent restenosis,10,11,21 the optimal stent design remains unknown. Our data suggest that the combination of fixed geometry, uniform cell size, and unique surface treatment of the Protea stent appears to impart a neointimal inhibition advantage in the porcine model. However, the potential clinical applicability and implications of our findings require further investigations.
In-stent restenosis is related in part to technical aspects of the device. Design features of the stent that influence outcome have been identified and optimised for improved performance. However, some current stents have significant contamination with industrial impurities on the surface and in the bulk. Previous research pioneered by Palmaz et al34 and more recently published by Sprague and colleagues35 suggest that even subtle differences in chemical composition of the stent material and in surface finish may influence the biological response. In specific, classic research by Schwartz and coworkers36 showed that contamination on a bare metal surface actually aggravates neointimal response and pressure rinsing of coronary stents immediately before implantation may reduce inflammation and neointimal hyperplasia. In our study, the Protea stent features a unique surface treatment eliminating most of the intrusions inherent to the CoCr alloy and it is very much possible that this interesting and innovative aspect of the Protea stent platform, and not only the thin struts and cell geometry, help achieve a superior biological response in comparison to Driver.
The implications of a superior stent platform design are obvious for both BMS as well as DES. Significant neointimal inhibition, as seen with the Protea, may potentially obviate the need for DES, thereby avoiding their attendant complications, in many clinical and anatomic scenarios. Moreover, this same attenuation of neointima may allow for decreased levels of drug coating on future DES, thus minimising potential vessel toxicities.
To sum up, design improvements such as thin struts, cell geometry, and ultra-smooth surface have probably contributed to the favourable preclinical outcomes with the Protea stent. Our results support the notion that apparently small improvements in basic but fundamental aspects of the BMS technology can result in actual biological benefits and ultimately pave the way to better DES platform.
Study limitations
The statistical analysis of data should be viewed in light of the relatively small numbers of test subjects. Longer-term studies are needed for verification and extension of these initial promising data. Additionally, as the Protea platform differs in both strut thickness, cell geometry, and surface finish from that of the control Driver stent, future evaluations utilising prototypes varying in only a single parameter may further elucidate the relative advantages of each feature. Of interest, however, the similar histologic responses of the single Protea layer versus overlapped segments suggest that strut thickness may not be the primary determinant for neointimal proliferation.
Conclusions
The ultra-thin-strut cobalt-chromium Protea stent, which features fixed geometry, uniform cell size, and ultra-smooth surface exhibited favourable arterial responses with significant reduction of neointimal formation in porcine coronary arteries, when compared to a commercially-available cobalt alloy BMS. This novel stent platform may represent an attractive alternative to current BMS designs. Our results showed how seemingly trivial improvements to the BMS technology can result in substantial biological responses. Further, long-term trials are needed to assess the potential clinical applicability and impact of present findings.

