Abstract
Aims: The Svelte Stent-On-A-Wire (SOAW) is a thin strut novel device consisting of a balloon-expandable cobalt-chromium stent premounted onto a single lumen fixed-wire delivery catheter platform. We evaluated the performance of the novel Svelte SOAW in comparison with the MultiLink Vision (ML Vision) balloon-expandable stent, in porcine coronary arteries.
Methods and results: Eight Yorkshire swine (30-day follow-up cohort) and eight Yucatan mini-swine (90-day follow-up cohort) were implanted with either Svelte or control ML Vision. Acute performance characteristics were graded by interventionalists during implantation. Angiographic assessments were performed at the index procedure and at 30 or at 90 days post implantation. Scanning electron microscopy (SEM), histological and histomorphometric analysis of stented segments were performed after angiographic follow-up. Acute implantation performance was similar between the two stents; however, deflation time was significantly lower in the Svelte stent group (Svelte 4.70±0.93 s vs. ML Vision 9.56±0.96 s, p <0.05). Angiographic late loss was similar for both stents at 30 (Svelte 0.83±0.59 mm vs. ML Vision 0.88±0.71 mm, p=0.969) and at 90 days (Svelte 0.76±0.35 mm vs. ML Vision 0.83±0.35 mm, p=0.679). SEM analysis showed complete endothelialisation at 30 days in both stent types. Histopathological assessment demonstrated minimal injury and inflammation at 30 and 90 days with Svelte and ML Vision stents as well as similar endothelialisation, neointimal maturation, adventitial fibrosis and neointimal fibrin. No evidence of in-stent thrombus was reported in either stent group. Histomorphometric analysis showed no differences between the two groups in lumen, stent, media or neointimal areas at either 30 or 90 days post implantation.
Conclusions: At 30 and 90 days after implantation in porcine coronary arteries, the Svelte Stent-On- A-Wire showed vascular healing and tissue response equivalent to that observed with ML Vision stent.
Introduction
The Svelte Stent-on-a-Wire (SOAW) (Svelte Medical Systems, Inc., New Providence, NJ, USA) is a thin strut novel device consisting of a balloon-expandable cobalt-chromium stent premounted onto a single lumen fixed-wire delivery catheter platform.
The Svelte SOAW Delivery System includes a reshapeable platinum/iridium coil tip, a high-pressure nylon balloon, a stainless steel core wire, proximal and distal stent elastomer bands with a polymer tube distal shaft. The system has a 0.012” lesion entry profile and is compatible with 5 Fr or larger guiding catheters.
Fixed-wire (FW) balloon systems have been previously introduced in the attempt to provide lower profile systems allowing for treatment of complex lesions overcoming the over-the-wire devices available in the balloon angioplasty era.1,2 On the other hand, the development firstly of lower profile over-the-wire balloon systems and secondly of coronary stents resulted in the abandonment of the fixed-wire balloons.
The Svelte SOAW system is a new generation of fixed-wire stent technology that was specifically designed and developed for a direct stenting use3. Direct stenting is currently employed in relevant number of PCI procedures.4,5 This technique was demonstrated, in several studies using bare metal stents, to reduce procedural time, radiation dose, contrast administration, and costs in selected populations.6-14 The present device could provide a tool to reduce even more radiation exposure, contrast and procedural time not requiring the guidewire placing.
However, no large data are currently available on this technology regarding the vascular healing process after implantation and its performance in terms of angiographic results compared to other bare metal stents.
The present investigation is a pre-clinical study aiming at acquiring preliminary information on vascular response after implantation of the new Svelte Stent-On-A-Wire compared with the MultiLink Vision stent (Abbott Vascular, Santa Clara, CA, USA) in the porcine model.
Methods
The present study was conducted at CBSET, Inc, Lexington, MA, USA in accordance with Good Laboratory Practices as defined by the United States Food and Drug Administration in addition to the American Heart Association guidelines for preclinical research and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1996).
Eight Yorkshire and nine Yucatan mini-swine were enrolled into the study; one Yucatan mini-swine was excluded prior to stent implantation due to unsuitable coronary arteries anatomy for implantation. Before assignment to the study, all animals were held in quarantine according to the Test Facility standard operating procedures.
Blood samples were collected from a peripheral vessel on day 0 prior to intervention, and on day 30, 60, and 90 (± 2 days) for haematology and serum chemistry analysis.
To prevent or reduce the occurrence of thrombotic events, animals were treated on day -1 or day -2 with aspirin (650 mg, per os) and clopidogrel (300 mg, per os). The animals were then treated with aspirin (81 mg, per os) and clopidogrel (75 mg, per os) daily.
Device implant procedures
Before starting the procedure Telazol (1:1 tiletamine hydrochloride and zolazepam hydrochloride 4-6 mg/kg, intramuscularly) was administered as a pre-anaesthetic. Pre-operative nifedipine (10 mg, sublingual) was also given.
The animals were then intubated and maintained under anaesthesia with isoflurane as an inhalant anaesthetic. Buprenorphine (0.01 mg/kg, IM) was administered at the time of induction and again every 4-12 hours for the first 36 post-operative hours. Additional doses of analgesia were administered under the direct supervision of the attending veterinarian for any animal showing signs of discomfort. Procedures were performed through the carotid or alternatively, femoral artery.
Heparin (100-150 U/kg), was administered, after placement of the introducer sheath, to prolong activated clotting time (ACT) to a target of approximately 275 seconds during device deployment.
Nitroglycerine (200 μg, intra-arterial) was administered before the angiograms. Svelte stents were deployed in up to two coronary arteries along with one control ML Vision stent in each animal. The balloon was then inflated at a steady rate to a visually assessed target balloon-artery ratio of 1.05:1-1.15:1 and held for 30 seconds. In both animal groups Svelte and ML Vision stents with diameters of 3.0 or 3.5 mm were implanted.
Acute performance evaluation
Acute performance characteristics were evaluated by the interventionist with a score of 3 considered average for marketed coronary stents (ML Vision).
5=excellent, 4=above average, 2=below average, and 1= unacceptable.
TIMI (Thrombolysis in Myocardial Infarction) flow scoring was 4= TIMI 3 (highest flow rate), 3=TIMI 2, 2=TIMI 1, and 1=TIMI 0 (no flow).
Quantitative coronary angiography
Late loss was calculated as the post-deployment vessel minimal lumen diameter (MLD) minus the pre-necropsy angiographic MLD. Percentage stenosis was calculated as the late loss divided by the post-deployment vessel diameter x 100. Balloon to artery ratios (ratio of balloon diameter during peak inflation pressure to the vessel diameter before stent placement) were calculated from the QCA measurements by dividing the deployed balloon diameter by the baseline vessel diameter.
The quantitative coronary analysis values were obtained using the Centricity Cardiology CA1000 Cardiac Review 1.0 QCA software.
Animal observations
After the terminal time-point angiography, animals were euthanised on days 30 and 90. Each animal was euthanised in accordance with accepted American Veterinary Medical Association (AVMA) guidelines.
Scanning electron microscopy (SEM)
The hearts containing stents for the SEM evaluation were perfused (pressure ~100 mm Hg) with lactated Ringer’s solution, until the effluent was non-sanguineous. A perfusion-fixation with 3.0% glutaraldehyde (GTH) was then performed. All hearts were stored in labelled GTH-filled containers and refrigerated until processing for SEM imaging. Excess adventitial tissue was removed from the explanted stented arteries.
Radiographs were used to determine stent location within the artery then the artery was bisected longitudinally and pinned onto a mountable surface to expose the luminal surface (Figure 1). These samples were rinsed with 0.1M sodium cacodylate buffer then post-fixed in 1% osmium tetroxide, and finally rinsed and stored in 0.1M sodium cacodylate buffer. Subsequently, stented arteries were dehydrated in a graded series of alcohol solutions, dried using a critical point dryer (CPD), mounted onto a metallic disk and ion sputter coated with metal.
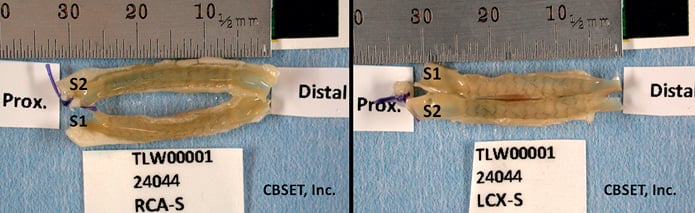
Figure 1. Stented coronary artery specimens. Stented artery dissected from hearts and bisected longitudinally, the luminal surface is exposed.
Specimens were photographed at low SEM magnification to create a surface map of the entire luminal surface to identify high-magnification imaging sites (Figure 2). SEM imaged vessels were investigated at each SEM imaged area and scored for:
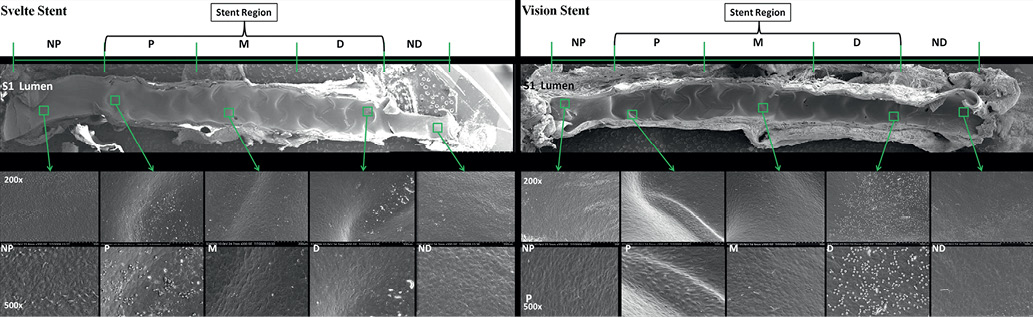
Figure 2. Scanning electron microscopy images at low and high-magnification at 30 days. All vessels showed 100% confluent endothelial cell with minimal intercellular spacing. Cell morphology was mostly transitional between polygonal and spindle shaped endothelial cells
Luminal endothelial coverage: (0=absence of endothelial cells; 1 <25% endothelial surface coverage; 2=25-75% endothelial surface coverage; 3 >75% endothelial surface coverage; 4=100% confluent endothelial cell coverage).
Endothelial confluence: (0=no intercellular spacing between endothelial cells; 1=minimal intercellular spacing; 2=notable intercellular spacing; 3=overwhelming intercellular spacing).
Endothelial morphology: (0=no endothelial cells observed per area examined [AE]; 1=predominantly squamous polygonal shaped endothelial cells per AE; 2=transitional morphology, mix of polygonal and spindle shaped endothelial cells per AE; 3=predominantly spindle shaped endothelial cells per AE).
Inflammation: (0=no inflammatory cells observed per AE ;1=minimal adhered inflammatory cells per AE; 2=notable adhered inflammatory cells per AE; 3=overwhelming adhered inflammatory cells per AE).
Thrombus: (0=no thrombus observed per AE;1=light adherence/deposition of thrombus material per AE; 2=notable adherence/deposition of thrombus material per AE; 3=overwhelming adherence/deposition of thrombus material per AE at the proximal, mid and distal segment of each longitudinal sectioned vessel).
Histology
All tissues/sections were processed using standard histological techniques.
Coronary arteries: Formalin-fixed stented vessels were carefully dissected from the myocardium. Transverse sections of unstented vessel were obtained within 5 mm of the proximal and distal edge of the stent. In addition, transverse sections were obtained from the proximal, middle and distal region of the stented segment of the vessel. All vessel sections were stained with haematoxylin and eosin (H&E) and a tissue elastin stain (e.g., Verhoeff’s).
Histopathology
Histopathological scoring via light microscopy was used to grade various parameters reflecting the degree and extent of the host response/repair process to treatment. These parameters included injury, inflammation, endothelialisation, and fibrin deposition.
The scoring of the arterial cross-sections was carried out as follows (Table 1):
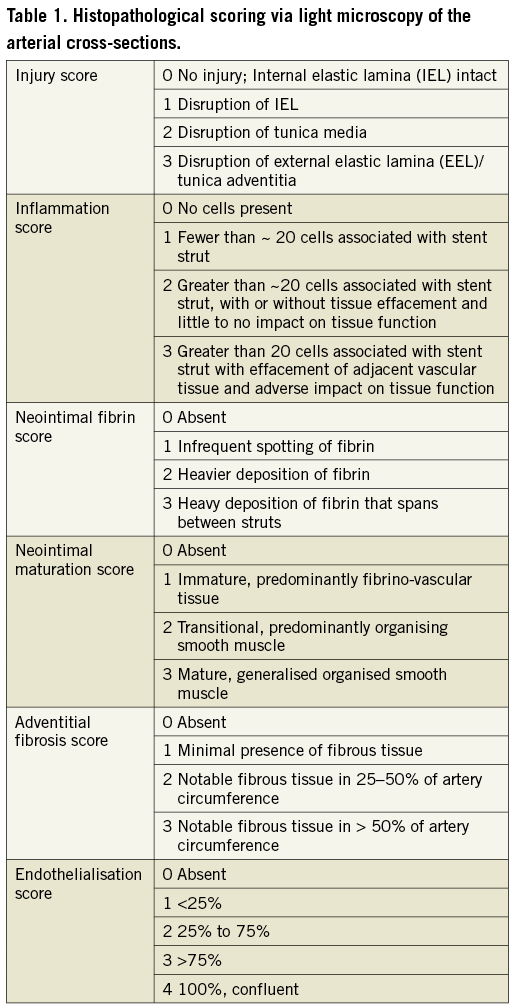
Injury score for stented arterial segments is dependent on that portion of the arterial wall disrupted by the stent and/or associated with tissue response. Injury was scored on a per-strut basis.
Inflammation score depends on the degree and extent of inflammation on a per-strut basis.
Neointimal fibrin score depends on the degree of fibrin deposition in the neointima.
Neointimal maturation score is determined by the relative amount of organised smooth muscle cells and fibrino-vascular tissue.
Adventitial fibrosis score depends on the severity of response and degree of circumference of artery affected.
Endothelialisation score depends on the extent of the circumference of the artery lumen showing coverage with endothelial cells. Scoring matrix for histopathology parameters is reported in Table 1.
Histomorphometry
Quantitative histomorphometric analysis was performed on the histological sections from each stented artery. For each histological section, the following parameters were directly measured using standard light microscopy and computer-assisted image measurement systems: lumen area (La), internal elastic layer bounded area (IELa), stent area (Sa), external elastic layer (EEL) bounded area. From these direct measurements, all other histomorphometric parameters were calculated.
Area measurements: Neointimal area (Na) as IELa - La mm2, medial area (Ma) as EELa - IELa, artery area (Aa) as La + Na + Ma.
Length measurements: Lumen diameter (Ld) as 2×√(La/π), IEL diameter (IELd) as 2×√( La + Na)/ π, stent diameter (Sd) as 2×√(Sa/ π), arterial diameter (Ad) as 2×√(Aa/ π).
Ratios: Lumen / artery areas (L:A) as La/ Aa, neointima / media areas (N:M) as Na / Ma, EEL / IEL areas (EELa: IELa) as Aa / (La+Na), IEL / stent areas (IELa:Sa) as IELa/Sa
Restenosis parameters: % area stenosis (% AS) as Na/(Na+La)× 100%, neointimal thickness (N) as (IELd- Ld)/2.
Histopathology - Myocardium
Light microscopy of myocardium harvested from regions subjacent and distal to stented vessels were used to determine whether there were any adverse reactions to stenting (e.g., infarction). Myocardium from regions adjacent and distal to non-stented vessels were used to assess pre-existing myocardial changes. All sections were stained with haematoxylin and eosin.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation and if normally distributed with homogeneous variances, were analysed by student’s t-test. If continuous data were not normally distributed or variances were not homogeneous, differences were assessed by Mann-Whitney rank sum test. Ordinal variables (scores) are presented as either means ±standard deviation and median (histopathology parameters) or median and interquartile range. Differences between categorical treatment parameters were assessed by Mann-Whitney rank sum test. For all statistical tests, the null hypothesis of no difference was only rejected if the value of the calculated statistic was less than 0.05 (p<0.05).
Treatment parameters were statistically compared using SigmaPlot 11.0.
Results
All data were generated at CBSET, Inc, Lexington, MA in cooperation with Svelte Medical, New Providence, NJ, USA.
Forty-one stents (16 Vision stents and 25 Svelte stents) were implanted in 16 animals.
One additional animal was enrolled but was not stented and excluded from the study due to unsuitable coronary artery size/anatomy.
In one animal, a Svelte stent migrated proximally during withdrawal of the balloon after deployment. The stent was repositioned and the balloon inflated to higher pressure with a good result. This animal was included in the 30-day SEM evaluation in order to have reliable data about the impact that this event could have on endothelialisation. There were no other procedural events or complications associated with implantation.
One animal on day one post-procedure, showed signs suggestive of gastrointestinal complications (irritation/ulceration). Clopidogrel and aspirin were discontinued and therapy for gastric ulcer was provided. When the animal became febrile, antibiotic therapy was instituted. The animal showed no clinical improvement and on day 4 was euthanised. Angiographic and histological assessment showed a stenosing aneurismal dissection in the proximal non-stented segment of the circumflex artery (Svelte stent) with moderate acute perivascular inflammation. Both the remaining implanted arteries in this animal (LAD, ML Vision stent; RCA, Svelte stent) were angiographically and histologically unremarkable.
Haematology, serum chemistry and fibrinogen
Baseline plasma fibrinogen was similar in both 30 day and 90 day treatment groups (180±19 mg/dL vs. 178±57, respectively p>0.05) and declined by 30 days (156±31.7 and 127±51 mg/dL for the 30 and 90 day groups, respectively). The animal that was euthanised moribund on day 4 (animal #61) had an elevated white blood cell count (26.5×103/μL; from 14.49×103/μL on day 0) and a substantially elevated fibrinogen concentration (558 mg/dL; from 314 mg/dL on day 0). The remaining animal haematology and serum chemistry values at 30, 60 and 90 days were within normal limits or did not deviate sufficiently to raise health concerns.
Acute performance evaluation
Overall the Svelte stent scored slightly better than the ML Vision control stent. Deflation time in particular was significantly lower for Svelte stents than for the ML Vision control stents (4.70±0.93 s vs. 9.56±0.96 s, p<0.05). However, for most measures of deployment, there was no difference between the Svelte and ML Vision stents (Table 2).
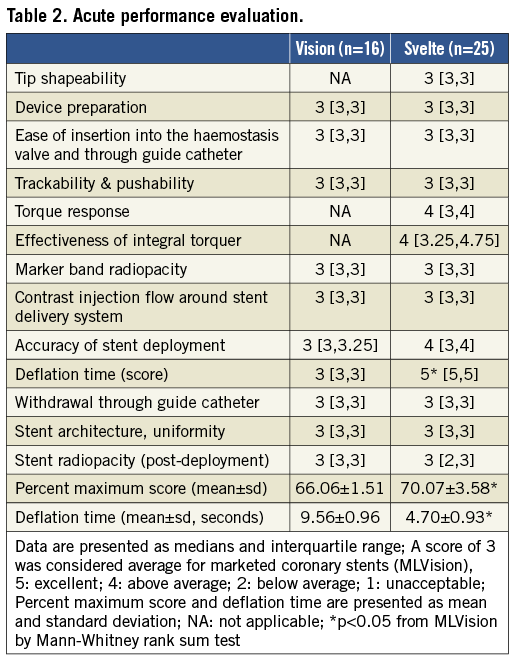
The ability to shape the fixed-wire tip was graded average compared to a standard coronary guidewire (Floppy BMW guidewire). Withdrawal of the delivery system was graded average with the exception of one Svelte stent which was scored unacceptable due to migration of the stent during balloon withdrawal.
Because the balloon to artery ratio was within a range that should have secured the stent to the vessel wall, it is most likely that the balloon pulled the stent back resulting in the unacceptable grade for this device. This did not occur in any other Svelte stent and was not considered typical for this delivery system.
Quantitative coronary angiography (QCA)
Baseline minimal lumen diameters (MLD) and balloon to artery ratio (B: A ratio) were similar for both stent treatments. At both 30 and 90 days after implantation, there were no significant differences in late loss and degree of stenosis between Svelte and ML Vision stents (Table 3). There was no angiographic evidence of dissection or perforation.
Scanning electron microscopy (SEM)
SEM analysis was conducted on three vessels at each 30 and 90 days (n=2 Svelte stents and n=1 ML Vision stent per time-point).
At 30 days, all vessels treated with both Svelte SOAW and ML Vision stent, showed 100% confluent endothelial cell coverage (Luminal endothelial coverage score 4) with minimal intercellular spacing (Endothelial confluence score 1) and cell morphology was transitional between polygonal and spindle shaped endothelial cells (Endothelial morphology score 2). Adherent inflammatory cells were rare (Inflammation score 1) and there was no evidence of thrombus (Thrombus score 0) (Figure 2).
At 90 days, all vessels showed 100% endothelial confluence cells with normal, polygonal morphology and a cobblestone pattern with tight junctions between cells. The presence of adherent inflammatory cells at 90 days was still minimal and equivalent for both stent treatments.
There was no in-stent thrombus in either stent group at any time point. Therefore, SEM analysis showed that vessels implanted with Svelte or ML Vision stents were fully healed by 90 days post stent implant.
Histomorphometry
No significant differences were observed between Svelte and ML Vision in artery, stent, lumen, media and neointimal areas at both 30 and 90 days post-implantation. Area percent stenosis and the artery ratios were also comparable for both stent treatments at 30 and 90 days. Vessel wall tissue response was therefore similar in both stent treatments (Table 4) (Figure 3 and Figure 4).
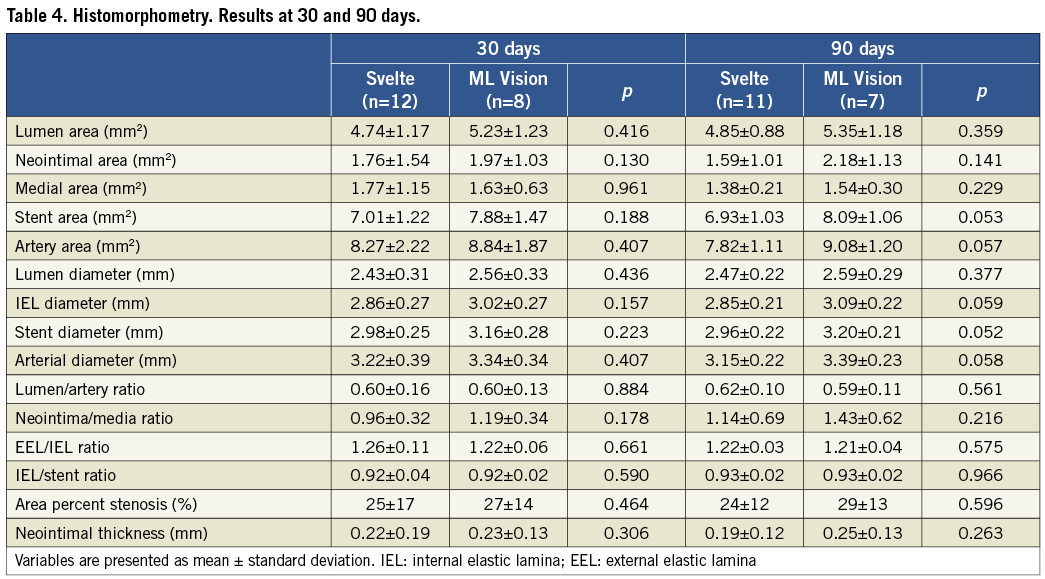
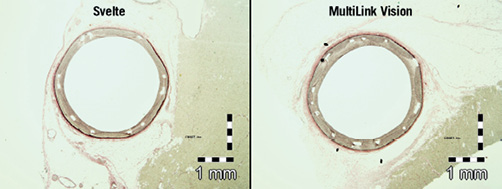
Figure 3. Histological transverse sections of stented vessel at 30 days. Area percent stenosis and the artery ratios were similar for both stent treatments.
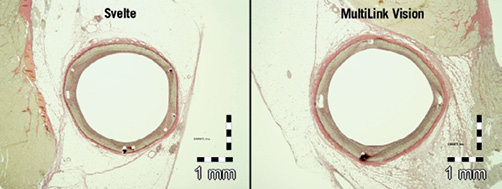
Figure 4. Histological transverse sections of stented vessel at 90 days. Tissue response was similar in both stent treatments.
Semi-quantitative histopathology
A summary of histopathology results is presented in Table 5 and frequency of score findings for selected parameters is in Table 6. Implantation of either Svelte or ML Vision stents resulted at both 30 and 90 days in negligible vessel wall injury and no significant differences between the two stent treatments. Results from injury assessment revealed that for both Svelte and ML Vision stent injury scores were low (0 or 1) in 92% and 97% of the struts respectively at 30 days and in 95% and 99% of the struts respectively at 90 days (Table 6).
Inflammation was also minimal for both groups and inflammation score was considerably less in the Yucatan mini-swine at 90 days compared to the Yorkshires at 30 days for both stent treatments demonstrating similar vascular tolerance. Assessment of frequency of the inflammatory scores revealed that for Svelte and ML Vision stent inflammation scores were low (0 or 1) in 83% and 91% of the struts respectively at 30 days and in 99% and 100% of the struts respectively at 90 days (Table 6).
Adventitial fibrosis was minimal and similar for both treatments at 30 days and 90 days.
Additional histopathological assessment showed complete endothelialisation by 30 days post-implant for both stent groups and a complete neointimal maturation in Svelte stents.
Neointimal fibrin was minimal throughout the study for both stent treatments (Table 5).
Regardless of group or time point, there were no significant findings associated with the proximal and distal non-stented portions of the stented arteries.
Myocardial findings
The most common observation was lympho-histiocytic cellular infiltrates. The infiltrate was characterised by minimal, focal to multifocal, accumulations of lymphocytes and histiocytes, occasional eosinophils, and rare multinucleated giant cells or neutrophils. Myocardial fibrosis or myofibre degeneration (i.e., putative areas of chronic “infarction”), were not observed.
There were no additional findings related specifically to the Svelte stent and no changes that were considered to be unusual or inconsistent with expected background levels.
Discussion
The present report is a preclinical study investigating the performance of the novel Svelte Stent-On-A-Wire compared to the ML Vision stent. The main findings are the following:
1) Svelte and ML Vision stent showed similar angiographic results at both 30 and 90 days after implantation in porcine coronary arteries with no differences in late lumen loss and percentage lumen stenosis;
2) Minimal vascular injury and inflammation were demonstrated in both stent treatments, and no differences were shown between the two stent groups in endothelialisation and neointimal maturation, which both appeared complete at 30 days:
3) Histomorphometric analysis demonstrated no differences between groups in neointimal growth and neointima/media ratio.
Previous reports demonstrated that in-stent restenosis is related to vascular injury15,16 and inflammatory response after stent implantation, as treated vessels develop neointimal tissue proportionally to the degree of both these parameters.17 As assessed by histopathology, the Svelte SOAW and the ML Vision stent showed both a low injury score at 30 days and inflammation was nearly absent at 90 days as no inflammatory cells or fewer than 20 cells per strut (histopathology Inflammation score 0 or 1) were detected in 99% of Svelte and 100% of ML Vision struts. These data were confirmed by SEM analysis with rare adherent inflammatory cells (SEM inflammation score 1) at 30 days for both stents.
On the other hand, a delayed and incomplete vascular healing, often associated with neointimal fibrin deposition18 and low neointimal maturation, might have a relevant impact on thrombotic events. In this regard, in the present report, neointimal maturation was complete in the Svelte SOAW group at 30 days and there was complete absence of neointimal fibrin in both stent-groups at 90 days. At the same time point, as assessed by SEM, all vessels showed 100% endothelial confluence cells with normal, polygonal morphology and a cobblestone pattern with tight junctions between cells. This complete endothelial coverage and neointimal maturation was associated with no in-stent thrombus detectable in either stent group at any time point.
These histopathological results showing a similar healing process, could explain the substantial equivalency of the two stent treatments at the histomorphometric analysis with no differences in neointimal area, neointimal thickness and neointima/media ratio.
Consistent with histopathological and histomorphometric data, quantitative coronary angiography demonstrated equivalent luminal losses and percentage luminal stenosis at 30 and 90 days for both stents.
The above-mentioned similar angiographic and histological results after implantation between the Svelte SOAW and the ML Vision stent could be partially due to the fact that the stent structure of the two study devices presents important similarities as they are both cobalt-chromium stents, with a similar strut thickness (0.0031” [SOAW] vs. 0.0032” [ML Vision]) and a similar strut width.
Furthermore, even if the two devices have a different stent delivery system, no differences were observed in trackability and pushability and a significant reduction in deflation time was assessed for the Svelte SOAW. Therefore, not only histological vascular response and angiographic results were comparable between groups but also the acute performance evaluation between the two delivery systems.
These preliminary results provide the first demonstration of the technical equivalency of the new Svelte SOAW with a commonly clinically used bare metal stent.
However, unlike other over-the-wire or monorail stents, the novel Svelte SOAW could show some additional advantages. A fixed-wire balloon-expandable stent could indeed provide a relevant innovation as this device is designed specifically for direct stenting, which has been demonstrated to reduce procedural time, radiation dose, contrast administration, and costs.13,14 Furthermore, the use of the Svelte Stent-On-A-Wire could theoretically provide additional savings not requiring the placement of a guidewire. These advantages could be clinically relevant, especially in patients with chronic kidney disease,19,20 advanced age21,22 and peripheral arterial disease.23,24
Another important characteristic of the present device is the substantially lower crossing profile than monorail or over-the-wire stents, as a very low profile could be relevant for stent-crossing during side branch treatment or in the complex lesions scenario (Figure 5).
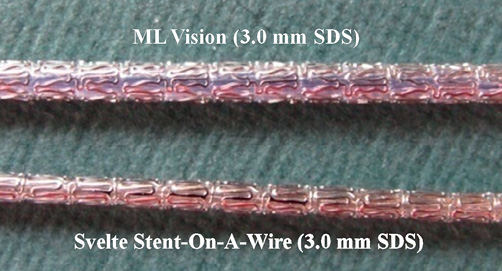
Figure 5. The Svelte SOAW crossing profile. The Svelte Stent-On-A-Wire shows a substantial lower crossing profile than standard stents.
The quantification of these theoretical advantages was not however per protocol planned in the present study and even a post hoc investigation would be affected by the fact that each animal was treated with both stents.
In conclusion, the present report is the first study demonstrating equivalent vascular response and angiographic results between the novel Svelte SOAW and a clinically used bare metal stent, suggesting that the implantation of the novel Svelte Stent-On-A-Wire is safe and feasible.
The analysis of the theoretical advantages of this new technology goes beyond the aims of this preliminary study and further clinical investigations are needed to observe the real benefit provided by this device. A currently on-going First-In-Man study could aid in further understanding about the performance of the Svelte SOAW in the clinical scenario.
Limitations
Different strains of pig were used for the 30-day time point (Yorkshire) and the 90-day time point (Yucatan), therefore comparisons and conclusions based on progression over time were limited. Scanning electronic microscopy was performed only in a limited number of coronary arteries and the results of this analysis should be considered exploratory and hypothesis generating. The present report is a preclinical study and data were acquired in healthy animal models thus not completely reflecting the human scenario with coronary artery atherosclerosis, calcified and complex lesions. Procedural time, radiation exposure and contrast medium delivered were not assessed and compared between stent treatments as per protocol each animal was treated with both devices.
Conclusion
Results of the present study demonstrated that the implant of the novel Svelte Stent-On-A-Wire is safe and feasible with a vascular response after implantation in porcine coronary arteries equivalent to that observed with ML Vision stent.
Acknowledgements
This study was funded by a grant from Svelte, Medical, New Providence, NJ, USA. All animal work, data, and images were generated by CBSET, Inc, Lexington, MA, USA.
Conflict of interest statement
W. Easterbrook and M. Pomeranz are employed by Svelte Medical Systems. G. Kopia is a paid consultant to Svelte Medical. As a partner in an independent and privately owned contract histopathology laboratory, S. Rousselle receives direct or indirect sponsorships from a variety of commercial entities, including Svelte and Svelte’s competitors. All the remaining authors have no conflict of interest to declare.

