Abstract
Aims: To evaluate the short-, mid- and long-term safety, efficacy and vascular physiology of Axetis silicon dioxide (SiO2, abrading the micropores) inert-coated stent implantation in a randomised preclinical setting.
Methods and results: Coronary arteries of domestic pigs were randomised to receive either Axetis or BMS (same design) stents with one-, three- and six-month follow-up (FUP), controlled by coronary angiography, optical coherence tomography (OCT), intravascular ultrasound (IVUS) and histology (n=32). The time-dependent vasomotor reaction of coronary arteries to stenting was measured using modified myography (n=12). Complete endothelialisation of the Axetis stent was confirmed by OCT, IVUS and histology at one-month FUP. Histopathology revealed continuous healing of the vessel wall with a gradual reduction of inflammation and fibrin score during the six-month FUP in both stent types. Significantly smaller neointimal area and %area stenosis were measured in Axetis stents compared with BMS at each FUP time point. Vascular reactivity measurements showed significantly better endothelium-dependent vasodilation of stented arteries with Axetis implantation.
Conclusions: Implantation of the Axetis SiO2-coated stent resulted in a significantly better safety, efficacy and vessel physiology profile compared with BMS of the same design with a continuous decrease in vessel inflammation during the six-month FUP.
Introduction
With an increasing number of drug-eluting stents (DES) being implanted worldwide, concerns about the long-term safety of DES have arisen, regarding delayed endothelialisation with late thrombosis and a consequent acute mortality rate of 45% to 75%1,2. Thus, especially in patients with relative contraindications to long-term clopidogrel treatment (e.g., higher risk of bleeding complications or absolute contraindication for long-term anticoagulation) or in patients with suspected incompliance, BMS might still provide an optimal choice3. Hence, attention has been focused on the structure and composition of stents, with particular interest in foreign body reaction of the vessel wall aiming to improve biocompatibility. A variety of different inert (non-drug-eluting) stent coatings, such as silicon carbide4, phosphorylcholine5 or heparin6 have been tested to provide a biological barrier between the stent surface, circulating blood and the endothelial wall, but they have not led to any clear reduction in restenosis or revascularisation in humans.
The chemical compound of silicon dioxide (SiO2) is generally used in medicine and in food production, due to its beneficial characteristics of electrical insulation and high chemical stability. The passive (inert) coating of a BMS with SiO2 has the advantage of electrically isolating the stent surface from the blood and its cellular components, along with the smooth nanocoating filling the micropores of the rough surface of a stent7. The Axetis stent (Axetis AG, Zug, Switzerland) is a novel SiO2 inert-coated stent which aims to replace the BMS with successful inhibition of neointimal growth, decrease of stent thrombosis and stent-related adverse cardiac events.
In this preclinical study of a porcine model of coronary stenting, we evaluated the feasibility, safety and efficacy of the Axetis stent by control angiography, intravascular ultrasound (IVUS), optical coherence tomography (OCT) and histomorphometry. Furthermore, we measured the vascular constriction and vasodilation responses of the coronary arteries after implantation of an Axetis or BMS stent.
Materials
AXETIS STENT PLATFORM
The platform of the Axetis inert-coated stent is the medical grade 316 LVM stainless steel balloon-expandable stent which has a tubular, open-cell design. Like the BMS, the Axetis stent is crimped on a semi-compliant balloon catheter. The passive coating of the stent consists of SiO2 with a coating thickness of 40-150 μm. The SiO2 coating has the capability to fill the pores of the stainless steel stent successfully, even the smallest ones which might catch platelets 2-4 µm in diameter (Figure 1).
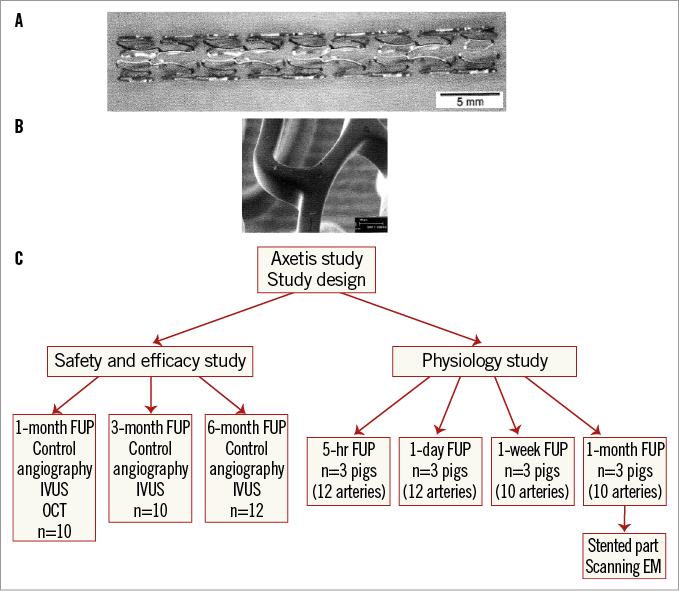
Figure 1. Stent design and study design. A) Stent design. B) Scanning electron microscopic image of the homogenous inert coating. C) Study design.
Methods
ANIMAL PREPARATION
Forty-four domestic crossbred pigs of both sexes (weight 20-25 kg), fed on a standard natural diet were included in the trial (n=32 for the safety and efficacy study, and n=12 for the in vitro vascular reactivity measurements) (Figure 1). After a loading dose of 100 mg acetylsalicylic acid and 300 mg clopidogrel per os 24 hours prior to stenting, coronary angiography, stent implantation and follow-up examinations were all carried out under general anaesthesia. Anaesthesia was initiated with 12 mg/kg ketamine-hydrochloride, 1 mg/kg xylazine and 0.04 mg/kg atropine and deepened with isofluorane and oxygen via a mask. Following intratracheal intubation, anaesthesia was maintained with the anaesthetic gas mixture of 2-3.5 vol% isofluorane, 1.6-2.5 vol% O2 and 0.5 vol% N2O. Blood pressure, O2 saturation and electrocardiogram were monitored continuously. The right femoral artery was prepared and 10,000 IU unfractionated heparin was administered.
The experiments were conducted in the Institute of Diagnostics and Oncoradiology, University of Kaposvar, Hungary, and approved by the Ethical Committee on Animal Experiments of the University of Kaposvar, Hungary, based on the “Principle of laboratory animal care” (NIH publication No. 86-23, revised 1985) and followed the guidelines for preclinical stenting8,9. The investigators were blinded regarding the stent types and coating.
CORONARY ANGIOGRAPHY AND STENT IMPLANTATION
After baseline coronary angiography in two projections, the stents were implanted randomly in the left anterior descending (LAD) and the left circumflex (LCx) arteries (one Axetis and one BMS in each animal). Implantation was carried out with an inflation time of 30 sec and a pressure of six to twelve atmospheres in order to reach a stent:artery ratio of 1.1:1. Only two stents were implanted in one pig and only one stent in each artery. After control angiography of the left coronary artery system, the guiding catheter and the introducer sheath were removed, the arteriotomy was ligated, and the skin was closed in two layers. The animals were then allowed to recover from the anaesthesia. Complications during and after stenting were noted and documented. The general health of the animals was observed daily.
FOLLOW-UP EXAMINATIONS
Follow-up (FUP) examinations included angiography and intravascular ultrasonography (IVUS) after four weeks (n=10), three months (n=10) and six months (n=12), with supplemental intravascular optical coherence tomography (OCT) (St. Jude Medical, St. Paul, MN, USA) at one-month FUP. During the FUP period the pigs received daily per os 100 mg acetylicsalicylic acid and 75 mg clopidogrel.
At one-month FUP after coronary angiography, an OCT imaging catheter (Dragonfly™; St. Jude Medical) was placed 3 cm distal to the stent in the stented artery and images were acquired during motorised pullback10. The images were captured and digitally stored by the ILUMIEN System (St. Jude Medical) for off-line analysis. The OCT imaging catheter was withdrawn and replaced by an IVUS catheter (Atlantis SR; Boston Scientific, Natick, MA, USA, available at the animal lab), and IVUS images were recorded during automatic pullback of 0.5 mm/sec for off-line qualitative and quantitative analyses.
Furthermore, stents were implanted for in vitro vascular reactivity measurements at 5 hrs (n=6 arteries of each BMS and Axetis), one-day (n=6 arteries of each BMS and Axetis) (time of acute stent thrombosis), one-week (n=5 arteries of each BMS and Axetis) (time to smooth muscle cell wandering to form neointima), and one-month FUP (n=5 arteries of each BMS and Axetis) (completeness of endothelialisation)11. The FUP time points of the in vitro measurements were chosen to catch the most important pathological changes leading to vessel thrombosis and hyperplasia of the neointima. After euthanasia, the hearts were explanted.
For histological analyses, the stented coronary arteries were flushed with physiological saline solution and pressure fixed (100-110 mmHg) in 4.5% formalin for thirty minutes. Then the arteries were prepared, explanted and fixed in formalin for 24 hours. The stented arteries were embedded in Technovit® 9100 (Heraeus Kulzer, Wehrheim, Germany).
For in vitro vasomotor measurements following the predefined FUP periods, the proximal segments adjacent to the stents were prepared and the measurements were performed immediately11. The stented sections were prepared for scanning electron microscopy (SEM) to visualise endothelialisation.
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA)
Using a computer-assisted QCA edge detection algorithm (ACOM.PC; Siemens AG, Munich, Germany) pre-stent, post-stent and FUP quantitative angiographic parameters were measured.
OCT
OCT measurements were performed using the Guidelines for OCT Imaging and Reporting10. Briefly, the qualitative and quantitative OCT measurements were performed using LightLab Imaging software (LightLab Imaging/St. Jude Medical). The sites of the analyses were the cross-sections with the smallest lumen area and the minimal lumen area (MLA), where the minimum (MLD) and the maximum lumen diameter were measured. The percent area stenosis (%AS=reference lumen–MLA/reference lumen) was assessed and the eccentricity lumen index at the MLA site was calculated as the (maximum lumen diameter–MLD)/maximum lumen diameter. Stenosis length was defined as the region around the MLA where the lumen area was ≥50% of the reference lumen area. The stent malapposition was defined as stent strut separation from the vessel wall by at least 150 µm distance. Thrombosis was defined as an irregular mass with dorsal shadowing, protruding into the lumen.
IVUS
The neointimal proliferation was analysed macroscopically by IVUS one, three and six months after stenting, in off-line mode with a computer-based IVUS analysis system (EchoPlaque 2; INDEC Systems Inc., Mountain View, CA, USA). After 3D reconstruction of the vessel, the lumen, stent, and external elastic membrane volume was measured. The volume obstruction was determined as the ratio of neointimal volume and stent volume, and expressed as percentage.
HISTOPATHOLOGY AND HISTOMORPHOMETRY
Histology was performed in all stented arteries which underwent IVUS and OCT imaging. SEM was performed in the arteries which underwent in vitro measurements (Figure 1). The histopathology and histomorphometry assessments were carried out by experienced observers without knowledge of the stent type. Sections from stent segments showing the most severe pathology variable measurement as determined by IVUS and from the adjacent proximal (10 mm proximal to the stent) and distal (10 mm distal to the stent) vessel segments were stained with toluidine blue, or haematoxylin-eosin and Movat’s pentachrome (separate staining for fibrin appearing bright red and collagen appearing gold-yellow).
Injury score, inflammation score, neovascularisation, fibrin deposition around the stent struts, haemorrhage, necrosis, and the degree of re-endothelialisation were evaluated on a scoring system ranging from 0 (minimal) to 3 (maximal). The endothelialisation was quantified by routine HE stained 4 µm thick histological section, according to the recommendation of the consensus group9,10. The percent amount of fibrin and collagen deposition was calculated, using computer-assisted planimetry (ImageJ 1.440; NIH, Bethesda, MD, USA) and related to the total area of the neointima. Histomorphometric parameters such as lumen, internal elastic lamina (IEL), media, external elastic lamina (EEL), adventitial and neointimal area, maximal neointimal thickness and percent area stenosis (%AS) (neointimal area/IEL area, expressed as percentage) were recorded for each segment. Positive (adaptive) remodelling index (EEL area of the stent body/EEL area of the proximal reference segment), negative (constrictive) remodelling index (EEL area of the stent body/EEL area of the distal reference segment), proximal edge effect (neointimal area at the proximal reference/neointimal area of the stent body) and distal edge effect (neointimal area at the distal reference/neointimal area of the stent body) were calculated.
IN VITRO EVALUATION OF VASCULAR RESPONSE TO STENTING
The 4 mm-long ring of non-stented arterial segments adjacent to the proximal stent edge was mounted on two L-shaped metal pins (0.4 mm in diameter) in a modified myograph11 in a 37°C temperature tissue bath containing a modified Krebs-Henseleit buffer. Endothelin (30 nM) was given to achieve a maximal vessel contraction (mN). Endothelium-dependent or endothelium-independent (stiffness of vascular smooth muscle after overstretch injury of PCI) vasodilation was induced by substance P (1 nM) or sodium nitroprusside (4 mM), and was expressed in percent change of contraction level in mN/s/mN units, respectively.
STATISTICS
Data were expressed as means±standard deviation. The one-, three- and six-month results were compared using ANOVA with repeated measures, supplemented by Student’s t-test if between-group comparisons were significant. The sample size was based on previously published data and accepted for preclinical coronary stent models8,9. Statistical significance was considered at p<0.05. Statistical analysis was performed with statistics software of SPSS for Mac, version 17 (SPSS Inc., Chicago, IL, USA).
Results
PROCEDURAL AND FOLLOW-UP DATA
The implanted stent length (20 mm of each), stent size (3.0 mm for one and three-month FUP and 3.1±0.8 mm for six-month FUP for both groups) and stent implantation pressure did not differ between the groups. The 2.5 mm-sized stent proved to be too small and slipped proximally in two cases, without further procedural complication. No other procedural complications occurred. During the one-, three- and six-month FUP, no events were recorded, and no “clinical” sign of stent thrombosis was suspected.
QCA RESULTS
The pre- and post-stenting QCA data were similar in the groups. No stent fracture was recorded and all stents were well expanded. A trend towards higher MLD and lower %DS was observed in the Axetis stent group compared with the BMS during all FUP periods (Figure 2).
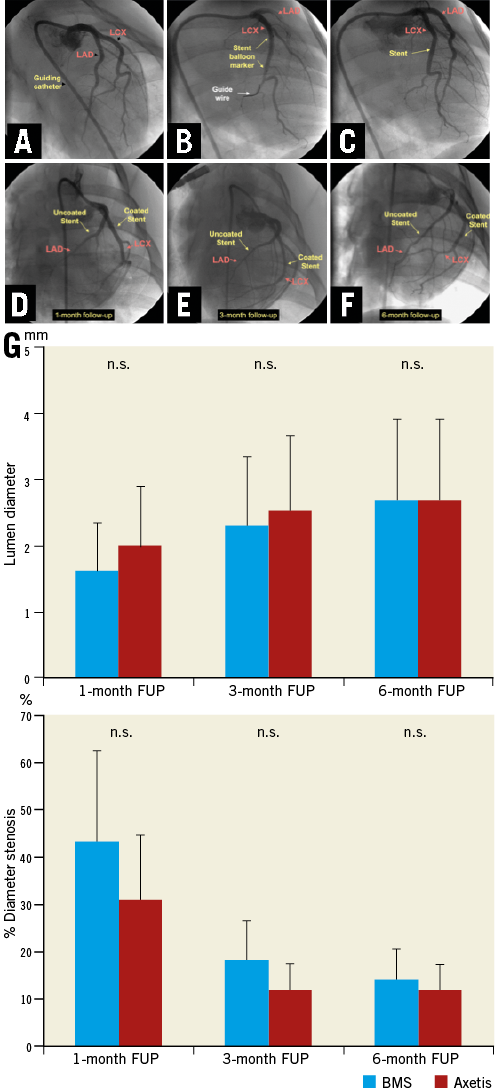
Figure 2. Baseline and follow-up angiography. A-C) Stent implantation in porcine coronary arteries. D-F) Representative one-month (D), three-month (E) and six-month (F) angiographic result of Axetis stent (coated) and bare metal stent (BMS) (uncoated) implantation. G) Results of quantitative coronary angiography.
IVUS RESULTS
Axetis stent implantation resulted in a non-significant decrease in neointimal volume and percent vessel obstruction for similar stent and vessel volumes as compared with the BMS (Figure 3). A gradual decrease in neointimal volume was observed during the one-, three- and six-month FUP.
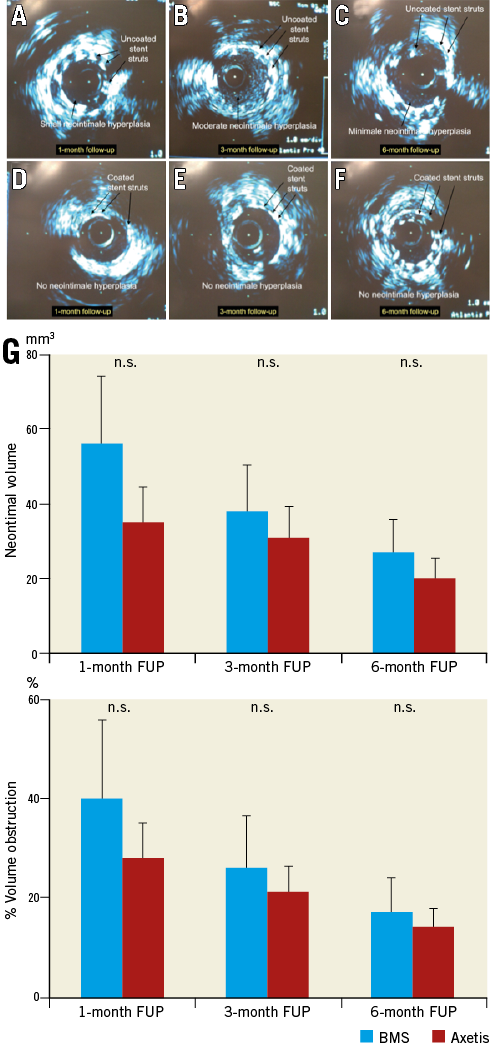
Figure 3. Representative intravascular ultrasound (IVUS) images of the Axetis stent (coated) and BMS (uncoated). A-F) One-month (A & B), three-month (C & D) and six-month (E & F) FUP IVUS of BMS (uncoated) and Axetis stent (coated). G) Results of quantitative intravascular ultrasound.
Due to the growth of the pigs during the three and six-month FUP, the vessel volume increased in both groups (152±16, 185±18 and 209±62 mm3 in the Axetis group and 167±21, 186±13 and 227±57 mm3 in the BMS group at the one-, three- and six-month FUP, respectively), with a virtual increase in the tissue volume outward of the stent.
OCT RESULTS
OCT revealed no malapposition or tissue protrusion, in-stent or edge dissection. No significant stenosis (%AS >50%) was measured. In accordance with the stenosis length definition, the stenosis length was zero for both stents (Figure 4).
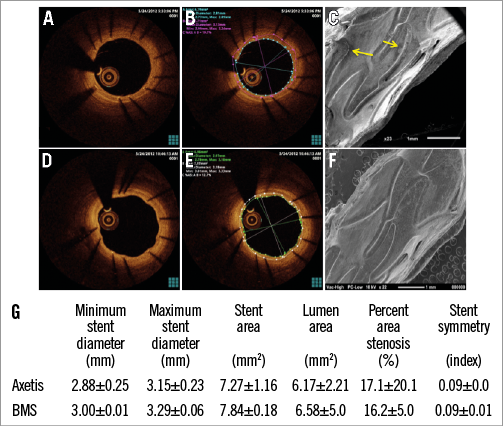
Figure 4. Representative pictures of BMS (A-C) and Axetis stent (D-F) by optical coherence tomography (OCT) and scanning electron microscopy (SEM) at one-month FUP. Native OCT picture of BMS (A) and Axetis stent (D) showing complete stent coverage with endothelium. Quantitative OCT measurements of BMS (B) and Axetis stent (E) at one-month FUP. Endothelial leakage of BMS (C) and smooth surface with unique endothelialisation (F) of Axetis stents by SEM. G) Results of quantitative OCT.
HISTOPATHOLOGICAL DATA
The injury score was similar in both groups. The endothelialisation was complete in all implanted Axetis stents (Figure 4, Figure 5). Two BMS stents showed partial endothelialisation at the one and three-month FUP. Endothelial recovery was also assessed by SEM in three stents of both Axetis and BMS at the one-month FUP, which confirmed the histological results (Figure 4). No necrosis around the stent struts was observed. Gradual decrease of fibrin and inflammation scores was measured with no difference between the groups (Figure 5). Fibrin and collagen deposits were mainly observed located adjacent to the stent struts. The % amount of fibrin was 7.7±3.6, 7.7±4.1 and 4.3±1.8% for Axetis and 8.6±4.3, 7.4±3.1 and 4.4±2.0% for BMS at one-, three- and six-month FUP. The % amount of collagen was 4.9±2.9, 5.1±2.0 and 5.7±2.5% for Axetis and 4.7±2.3, 5.1±3.0 and 5.8±2.7% for BMS at one-, three- and six-month FUP, respectively (Figure 6).
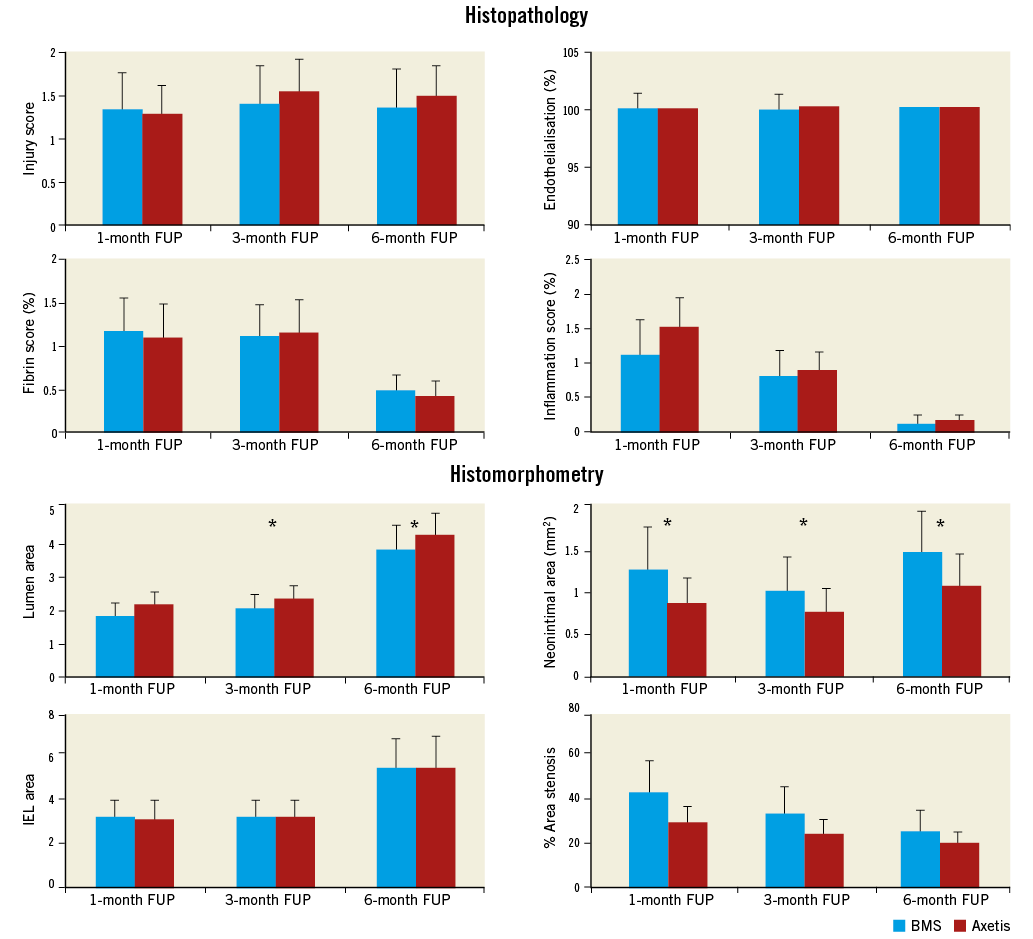
Figure 5. Time course of histopathological and histomorphometric parameters. Gradual decrease of the fibrin and inflammation scores during the one-, three- and six-month FUP by similar injury score. Significantly smaller neointimal area and %area stenosis using Axetis stent compared with BMS at each FUP. *p<0.05.
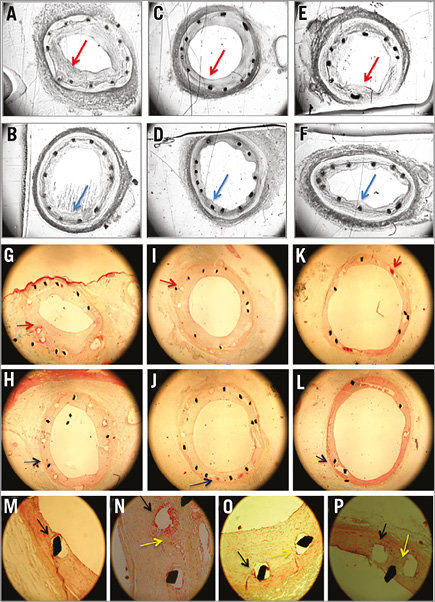
Figure 6. Representative histological images of the Axetis stent and BMS. Histology of stents (100 µm thick, Technovit embedding): A-F) greyscale of toluidine blue staining for histomorphometry, and G-P) Movat pentachrome staining for histopathology (4 µm thick). A-F) Histology of one-month (A & B), three-month (C & D) and six-month (E & F) FUP of BMS and Axetis stent, respectively, with small neointimal hyperplasia (red arrows for BMS and blue arrows for Axetis) (4x magnification). G-H) Fibrin deposition around the stent struts (red arrow for BMS and blue arrow for Axetis) at one-month (G & H), three-month (I & J) and six-month (K & L) FUP (4x magnification). M-P) Local inflammation (yellow arrow) and fibrin deposition around the stent (black arrow) and collagen fibres mostly adjacent to the stent struts (20x magnification).
Foreign body reaction was found in two Axetis stents with giant cells at the three-month FUP, which was probably the consequence of the unusually high degree of injury due to overdilation of the stents (injury score of 2.0 and 2.3), resulting in the complete rupture of the media and the penetration of the stent struts into the adventitia. Presence of some unifocal granulomas (1.77±3.58% vs. 1.6±2.2%) with some giant cells (1.35±1.18% vs. 1.16±2.06%), with no statistical difference between the Axetis and BMS stents, was found at the six-month FUP, where the initial injury scores were high (≥2).
HISTOMORPHOMETRY
Axetis stent implantation resulted in a significantly (p<0.05) higher lumen area and smaller neointimal area (0.88±0.40 vs. 1.29±0.58 mm2 at one month, 0.78±0.29 vs. 1.03±0.32 mm2 at three-month and 1.09±0.58 vs. 1.51±1.25 mm2 at six-month FUP) compared with BMS (Figure 5, Figure 6). Accordingly, the %AS was significantly (p<0.05) lower in the Axetis group compared to the BMS group (Figure 5).
Due to the growth of the pigs during the one-, three- and six-month FUP, the EEL area increased gradually (BMS: 5.71±0.43, 5.92±0.41 and 7.13±2.05 mm2, and Axetis: 5.74±0.52, 5.93±0.39 and 7.28±2.4 mm2, respectively), with no difference between the groups.
No clinically relevant proximal or distal edge effects were observed (Figure 7). There was no positive remodelling at the three and six-month FUP. Since the IEL area increased with no further thickening of the neointima, the calculated %area stenosis decreased.
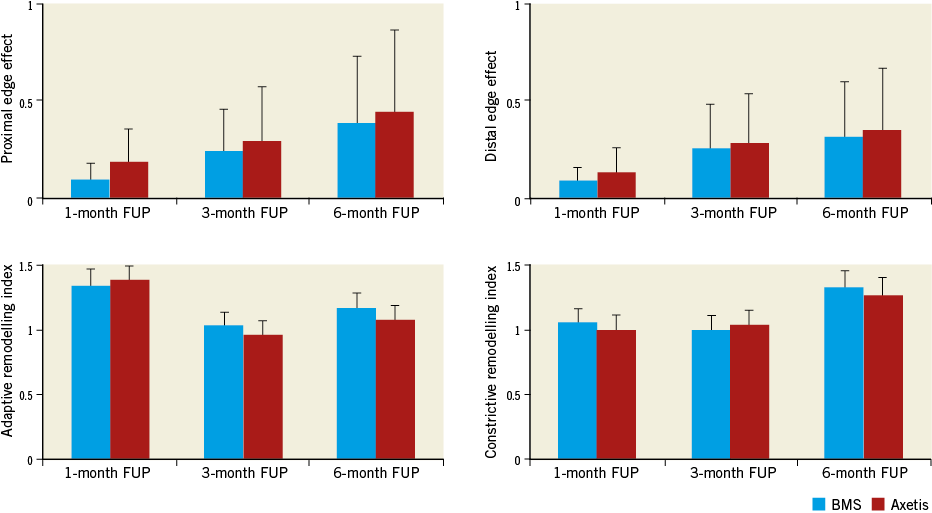
Figure 7. Time course of adaptive and constrictive remodelling and edge effects as measured by histomorphometry. No differences between the groups. No meaningful edge effects or adaptive or constrictive remodelling (for definition see Methods).
VASCULAR RESPONSE TO STENTING
Coronary arteries of both stent types showed susceptibility to vasoconstriction early (5 hrs) after stenting, which decreased at the one-day FUP, and remained in the normal range during the entire FUP (Figure 8).
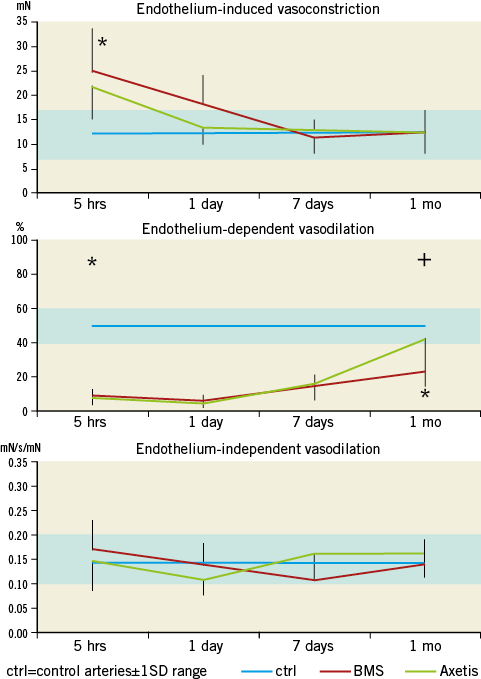
Figure 8. Time-dependent vasoconstriction and dilation post stenting. A) Significantly enhanced endothelin-induced vasoconstriction post stenting with either Axetis or BMS with no difference between the stents. *p<0.05. B) Profound and long-lasting impairment in endothelium-dependent vasodilation induced by substance P with only partial recovery of the BMS-stented arteries. *p<0.05 between controls and stents. +p<0.05 between Axetis and BMS. C) Endothelium-independent vasodilation by sodium nitroprusside is not affected by implantation of BMS or Axetis stents. The blue rectangle represents the normal range (non-instrumented arteries) (mean value±1SD)11.
The endothelium-dependent vasodilation was profoundly impaired after PCI for both stent types, indicating endothelial injury post stenting with slow recovery during FUP. At one month, the inert-coated Axetis stent showed a significantly (p<0.05) better endothelium-dependent vasodilation capacity compared to the control BMS.
No change in endothelium-independent (media muscular layer-dependent) vasodilation was observed during the FUP.
Discussion
Our study supported the feasibility, safety and efficacy of the implantation of the newly designed Axetis stent with SiO2 inert coating. The long-term (up to six-month) FUP found significantly better histological and physiological outcomes for Axetis stents in terms of neointimal proliferation or improved endothelium-dependent vasodilation compared with BMS.
FUP PERIOD
During FUP, no “clinical event” such as sudden death or haemodyamic compromise occurred, indicating no suspected acute (within 24 hrs post stenting), subacute (during one month of FUP) or late stent thrombosis or any other incidents with either the BMS or the Axetis stents. All pigs received dual antiplatelet therapy during the entire FUP period, which could be terminated after one month in case of the BMS and passive coating. However, the observers were unaware of the type of “coating” (passive or drug-eluting) or type of stent (Axetis or BMS), and similar medical treatment was maintained in both groups.
BMS AND INERT-COATED STENTS
With conventional BMS, the in-stent restenosis (ISR) rate is estimated to be up to 40%4, which did not improve using inert-coated stents12. However, a passive coating below a drug-elution system might result in better life-long biocompatibility after degradation of a drug-carrier polymer and elution of a drug (e.g., everolimus-coated DES with biodegradable polymer, XIENCE; Abbott Laboratories, Abbott Park, IL, USA)13.
The Axetis inert-coated stent exhibited good biocompatibility, with no clinical, angiographic, OCT or histological signs of stent thrombosis. Histological analyses of the different FUP series revealed continuous healing of the arterial wall, expressed as a significant reduction in inflammation and fibrin scores in both BMS and Axetis stents, but significantly smaller neointima with higher lumen area at all FUP periods when using the Axetis stents. Since the amount of the neointima was relatively low, the progressive accumulation of matrix elements and collagen was also relatively low compared with literature data14,15. Accordingly, no meaningful adaptive or constrictive remodelling was observed. This might be the reason for the discrepancy between our results and the findings of human atherectomy samples of in-stent restenosis16 or histology of high-degree in-stent neointimal proliferation of porcine coronary arteries17. The edge effect (index independent from the vessel growth) increased moderately with time without reaching clinical relevance. Since the stent size remains constant even if the arteries of the juvenile pigs shows moderate enlargement, the decrease of %AS within the stent presents the relative decrease of neointima, as also observed by other authors18.
FUNCTIONAL VASOMOTOR RESPONSE OF THE ARTERIES AFTER STENTING
Previously, we have shown impaired vasomotor reaction after the use of balloons, BMS and DES11. Similar to our previous findings, the arteries have shown affinity to contraction early post-PCI with rapid normalisation regarding endothelin-induced vasoconstriction. The behaviour of the Axetis inert-coated stent arteries was similar to that of the BMS arteries, but differed from that of the drug-eluting systems (balloons and DES) used in our previous study11. In accordance with the profound injury of the local endothelium by stenting, the endothelium-dependent vasodilation was impaired early post-PCI, with slow recovery. However, at one month we could confirm the normalisation of the endothelium-dependent vasodilation in the arteries with Axetis stents.
Limitations
Intracoronary devices may induce acute vessel thrombosis19 and iatrogenic denudation of the arteries. However, similar to other studies20, we saw neither acute thrombus formation nor denudation of the arteries, during the imaging and in the histology.
We have considered the growth of the domestic pigs in our long-term study, as indicated in the current guidelines recommending also the use of minipigs as the animal model for preclinical studies8,9. However, crossbred pigs may also be useful in a long-term study, if a timely comparison or time course of angiographic and histologic parameters is preferred, as demonstrated in previous publications18,21. Additionally, the increase of the EEL remained within a comparable range during the six-month FUP, and the expansion of the EEL was not associated with meaningful positive remodelling in our study (Figure 7).
Conclusion
Implantation of the Axetis SiO2 inert-coated stent proved to be safe and effective without stent thrombosis or other adverse events, and there was a low degree of neointimal proliferation with complete endothelialisation during the first month post implantation. Inert coating did not worsen the pathophysiological response of the coronary arteries subjected to PCI, showing similar endothelin-induced vasoconstrictive response compared to BMS, but it led to a faster recovery of the endothelium-dependent vasodilation capacity of the PCI-instrumented arteries.
| Impact on daily practice With an increasing number of implanted drug-eluting stents (DES) in coronary arteries, concerns about the long-term safety of DES have arisen. Therefore in patients with relative or absolute contraindications for long-term dual antiplatelet treatment or in patients with suspected incompliance, bare metal stents (BMS) might still provide an optimal choice. The passive (inert) coating of a BMS with SiO2 has the advantage of electrically isolating the stent surface from the blood and its cellular components, along with the smooth nanocoating filling the micropores of the rough surface of a stent. The Axetis stent is an inert-coated stent aiming to replace the BMS with successful reduction of neointimal growth and decrease of stent thrombosis, resulting in decreased stent-related adverse cardiac events. |
Funding
The study was sponsored in part by Axetis AG, a spin-off company of the University of Zürich. The blinded data analysis was supported by the Ludwig Boltzmann Institute Cluster Cardiovascular Research.
Conflict of interest statement
S.P. Hoerstrup is a consultant of Axetis AG, Germany. The other authors have no conflicts of interest to declare.

