
Abstract
Aims: The aim of this study was to assess the acute performance of the 95 µm ArterioSorb oriented poly L-lactic acid (PLLA) scaffold in comparison with the XIENCE metallic drug-eluting stent (DES) in porcine coronary arteries.
Methods and results: In 15 non-atherosclerotic Yucatan mini pigs, the ArterioSorb (3.0/14 mm) and XIENCE (3.0/15 mm) were implanted in 25 and 15 vessels, respectively. Acute performance was evaluated by using quantitative coronary angiography (QCA) and optical coherence tomography (OCT). Following three-dimensional reconstruction of the coronary arteries, endothelial shear stress (ESS) was quantified using non-Newtonian steady-flow simulation. Acute recoil measured by QCA was comparable in the two arms. Post-procedural flow and scaffold/stent area by OCT did not differ between the two devices. ESS post procedure was comparable between ArterioSorb and XIENCE (2.21±1.97 vs 2.25±1.71 Pa, p=0.314).
Conclusions: Acute recoil, luminal dimensions and ESS in the ArterioSorb oriented PLLA scaffold with thin struts of 95 µm were comparable to those in the XIENCE metallic DES.
Introduction
Bioresorbable scaffolds (BRS) were developed with the hope that they would overcome the long-term complications observed with metallic drug-eluting stents (DES) including delayed vessel healing, hypersensitivity reactions, restenosis, and neoatherosclerosis with the risk of repeat intervention and stent thrombosis (ST). However, the Absorb™ (Abbott Vascular, Santa Clara, CA, USA), the most investigated BRS, was shown to have an increased risk of target lesion failure in comparison with a contemporary everolimus-eluting metallic DES (XIENCE; Abbott Vascular)1.
The ArterioSorb™ scaffold (Arterius Ltd, Leeds, United Kingdom) was developed as a second-generation BRS using a patented die-drawing processing technique of poly L-lactic acid (PLLA) that results in improved radial strength, allowing reduced strut thickness. This results in a low crossing profile and is likely to reduce the occurrence of in-device thrombosis caused by bulky struts disrupting the arterial blood flow (i.e., endothelial shear stress [ESS]).
The aim of this study was in vivo assessment of angiographic and optical coherence tomography (OCT) results, and ESS distribution following ArterioSorb PLLA scaffold and XIENCE metallic DES implantation in the coronary arteries of Yucatan mini pigs.
Methods
STUDY DEVICES
The ArterioSorb scaffold comprises an oriented PLLA backbone with a strut thickness of 95 μm, coated with a layer of poly (D, L-lactic acid) (PDLLA) eluting rapamycin (1.45 μg/mm2) (Supplementary Figure 1). The ArterioSorb is manufactured using melt processing (extrusion) and die-drawing (solid-phase orientation) techniques. The strut width of the ArterioSorb is 170 µm.
The control everolimus-eluting durable polymer XIENCE stent is a cobalt-chromium alloy device with a strut thickness of 89 μm. The strut width of the XIENCE stent is 81.3 µm.
The in vitro study demonstrated that the mechanical properties of the ArterioSorb were equivalent to the metallic DES (Supplementary Appendix 12, Supplementary Figure 2, Supplementary Figure 3).
STUDY DESIGN
Fifteen non-atherosclerotic Yucatan mini pigs were included. The ArterioSorb (3.0 mm diameter × 14 mm length) and the XIENCE (3.0 mm diameter × 15 mm length) were used. When possible, an ArterioSorb or XIENCE was implanted in the three coronary arteries (right coronary artery, left anterior descending artery, and left circumflex artery), depending on the suitability of the vessel diameter measured by preprocedural angiography and OCT. The types of device implanted were balanced among the three coronary arteries. A maximum of four devices per animal were implanted. Y. Onuma attended the experiments. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the testing facility (AccelLAB Inc., Boisbriand, Quebec, Canada). The review ensured compliance with Canadian Council on Animal Care regulations. The testing facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and the Canadian Council on Animal Care (CCAC).
PROCEDURE DETAILS
A scaffold/stent was deployed in a segment of coronary artery free from side branches, whenever possible. Post-dilatation using a non-compliant balloon catheter was performed to achieve a targeted balloon-to-artery ratio of 1.1:1 and to ensure good apposition and embedment of the scaffold/stent struts under OCT guidance.
DATA ACQUISITION AND QCA/OCT ANALYSIS
The specific details of the data acquisition and quantitative coronary angiography (QCA) and OCT analyses are provided in Supplementary Appendix 2,3,4,5.
SHEAR STRESS ANALYSIS
The detailed methods of three-dimensional reconstruction of the coronary artery and subsequent computational fluid dynamics (CFD) analysis are included in Supplementary Appendix 3,6,7,8,9,10,11,12,13.
STATISTICAL ANALYSIS
The statistical analysis methods are described in Supplementary Appendix 4,13.
Results
PROCEDURAL CHARACTERISTICS
Procedural characteristics are shown in Supplementary Table 1. Maximal pressure during device implantation or post-dilatation was not statistically different between the ArterioSorb and XIENCE devices. Post-dilatation was performed in all scaffolds in the ArterioSorb arm, whereas in the XIENCE arm 80% of stents were post-dilated (p=0.046).
ACUTE PERFORMANCE
Periprocedural QCA measurements are shown in Supplementary Table 2. Device balloon-artery ratio as well as post-dilatation balloon-artery ratio were comparable between the two arms. Post-procedural mean or minimum lumen diameter was similar in the two arms. Figure 1 shows serial changes in mean lumen and balloon diameter during the procedure. Absolute recoil after device deployment and the one after post-dilatation were comparable between ArterioSorb and XIENCE.
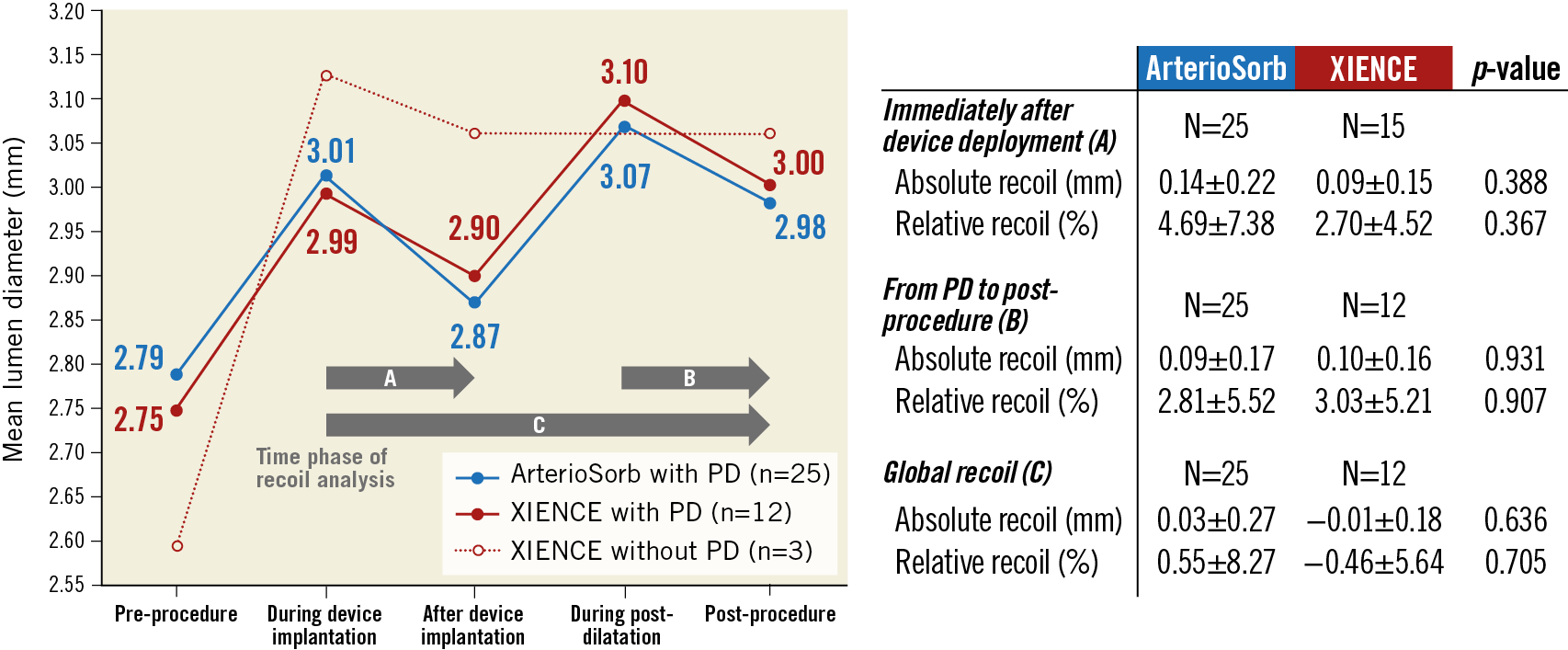
Figure 1. Recoil assessment by quantitative coronary angiography during procedure. The graph lines show serial changes in mean lumen diameter in device segments. PD: post-dilatation
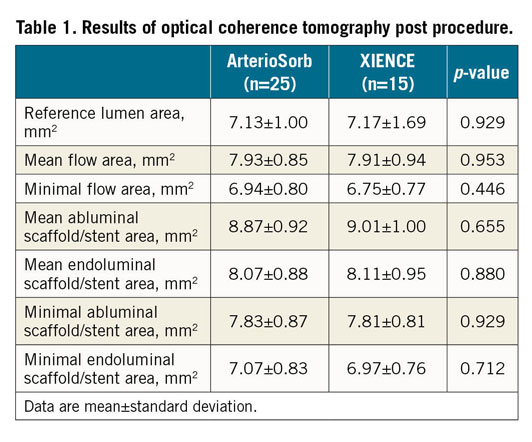
Table 1 shows that quantitative OCT results post procedure did not differ significantly between ArterioSorb and XIENCE.
SHEAR STRESS COMPARISON BETWEEN ARTERIOSORB AND XIENCE
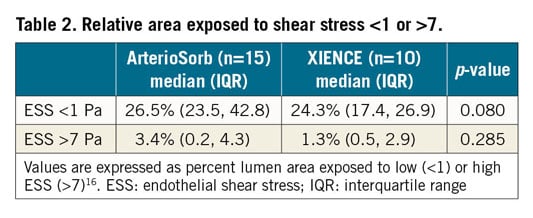
Supplementary Figure 4 shows a study flow chart for the CFD analysis. There were 10 scaffolds and five metallic stents excluded from the shear stress analysis due to technical reasons (i.e., absence of landmarks) in ArterioSorb and XIENCE, respectively. Finally, 134,856 five-degree sectors in 15 ArterioSorb scaffolds and 104,328 sectors in 10 XIENCE stents were included in the statistical analysis. Figure 2 shows cumulative frequency curves of ESS in five-degree sectors in cross-sections. ESS post procedure was comparable between ArterioSorb and XIENCE (2.21±1.97 vs 2.25±1.71 Pa, p=0.314). In contrast, relative area exposed to low ESS (<1 Pa) was larger in ArterioSorb than in XIENCE, although this did not reach statistical significance (Table 2).
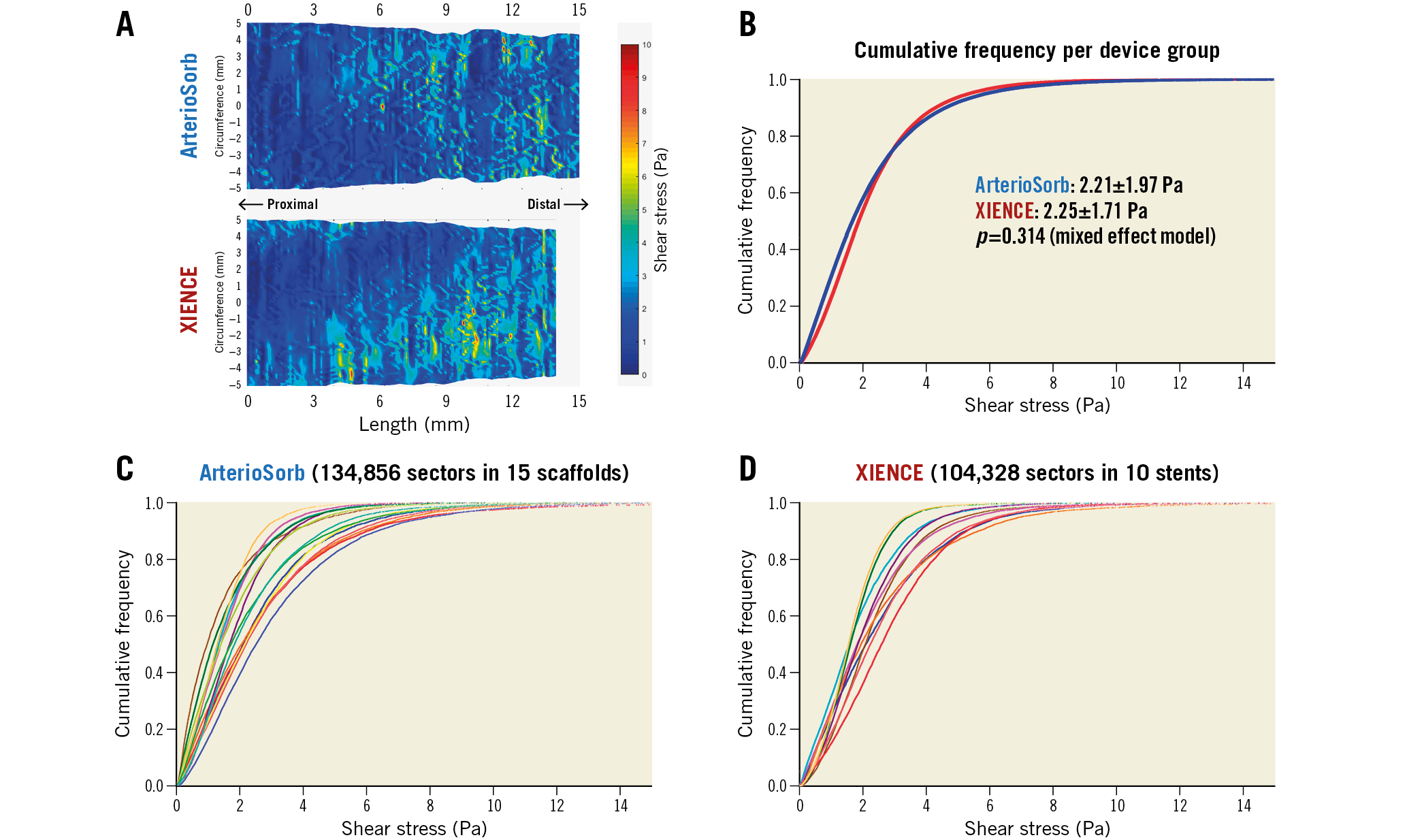
Figure 2. Comparison of shear stress in the ArterioSorb BRS and XIENCE DES. A) Spread-out maps of representative cases in ArterioSorb (above) and XIENCE (bottom). B), C) & D) Cumulative frequency curves of ESS values in five-degree sectors per device group, each case in ArterioSorb, and each case in XIENCE, respectively. Note that the cumulative frequency curves per device group were almost superimposed. BRS: bioresorbable scaffold; DES: drug-eluting stent
STRUT PROTRUSION AND SHEAR STRESS
Mean strut protrusion was higher in ArterioSorb than in XIENCE (72.6±10.2 vs 42.6±13.7 µm, difference 29.9 µm [95% confidence interval: 27.1 to 32.8], p<0.001). The relationship between strut protrusion and ESS is shown in Figure 3. A significant linear relationship was observed between strut protrusion and relative area with shear stress <1 Pa (plotted per cross-section) in ArterioSorb but not in XIENCE. An exponential curve [thick grey line, y=(5.3e-3)*exp(4.2e-2*x) + 0.19, R2=0.130] was fitted for the overall population, suggesting that strut protrusion of less than 60 µm does not affect shear stress.
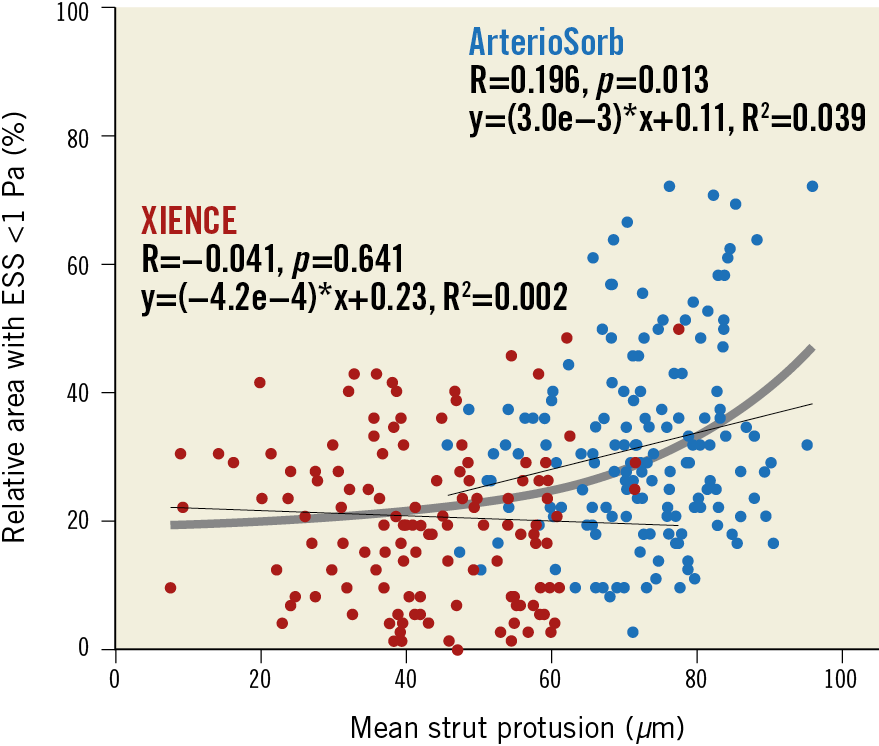
Figure 3. Relationships between strut protrusion and relative area with low shear stress. ESS: endothelial shear stress
Discussion
The main findings of this study are the following. 1) QCA analysis demonstrated similar mean and minimal lumen diameters post procedure in ArterioSorb and XIENCE. Recoil profiles in ArterioSorb after device implantation and after post-dilatation matched those of XIENCE. 2) Post-procedural OCT confirmed similar post-procedural luminal and device dimensions in the two groups. 3) CFD analysis demonstrated comparative ESS between the two devices.
This oriented polylactide polymer rendered the manufacture of thinner struts possible without loss of radial force and without increase in recoil. Acute recoil analysis by QCA in comparison with XIENCE using the same animal indicates that the recoil post implantation or post-dilatation is comparable.
Previous studies showed that thick BRS struts result in low ESS of the device segment13. Thin struts protrude less in the lumen and create fewer low ESS regions that are known to trigger and promote neointimal hyperplasia and neoatherosclerosis7,14. In the present study, ArterioSorb, with its reduced strut thickness of 95 µm, close to that of the XIENCE (89 µm), demonstrated comparative ESS to the XIENCE device. While there was a non-significant increase in the relative area exposed to low ESS (<1 Pa) in ArterioSorb as compared to XIENCE in the present analysis, a previous in vitro experiment suggested there is no significant difference in thrombogenicity between the 95 µm ArterioSorb and the XIENCE, whereas thrombogenicity in 150 µm BRS was significantly higher than in these two devices15.
Our analysis showed that strut protrusion below a threshold of 60 µm does not affect shear stress (Figure 3). If the thickness of a strut came close to 60 µm, there would be no significant differences in the shear stress distribution amongst devices.
Following the present study showing comparative mechanical performance and CFD results of the ArterioSorb BRS as compared to the XIENCE DES, planning of a first-in-man trial is underway.
Study limitations
This is an acute phase study demonstrating the acute performance of a novel bioresorbable scaffold. Follow-up evaluation by QCA, OCT and histology will unravel the device behaviour over time. Further preclinical evaluation will be required to assess mechanical behaviour and resorption profile. The study was performed in a non-atherosclerotic porcine model; therefore, the effect of atherosclerosis on the performance of the device cannot be assessed.
Conclusions
Using oriented PLLA, radial strength is considerably increased and thin struts of 95 µm are made possible. In a porcine model, ArterioSorb scaffolds demonstrated comparable luminal dimensions and shear stress post procedure in comparison with XIENCE best-in-class DES.
|
Impact on daily practice A thin-strut bioresorbable scaffold with oriented PLLA is expected to have better mechanical properties than conventional PLLA scaffolds, and equivalent ones to metallic DES. Using oriented PLLA, the radial strength of the 95 µm ArterioSorb BRS was considerably increased and equal to the XIENCE DES, resulting in comparable QCA and OCT results as well as similar endothelial shear stress. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
This study was funded by Arterius Ltd, Leeds, United Kingdom. M. Tomaniak acknowledges funding received from the European Society of Cardiology in the form of an ESC 2018 grant.
Conflict of interest statement
J.J. Piek receives non-financial support (travel costs for meetings and educational symposia) from Abbott Vascular as a former member of the medical advisory board. N. Bullett is an operations manager at Arterius Ltd. N. Ahmed is a development scientist at Arterius Ltd. K. Al-Lamee is a CEO/CTO at Arterius Ltd. G. Leclerc is a shareholder of AccelLAB Inc. P.W. Serruys reports consultancy fees from Abbott, Biosensors, Medtronic, Micell, Qualimed, Sinomedical Sciences, St. Jude Medical, Stentys, Svelte Medical Systems, Philips/Volcano, Xeltis, StentIt and HeartFlow. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

