
Abstract
Aims: In silico studies have provided robust evidence that stent design affects local haemodynamic forces, which appear as a major determinant of clinical outcomes following stent implantation. However, the implications of different stent/scaffold configurations on local haemodynamic forces have not yet been investigated in vivo in a comparative fashion. The aim of this study was to compare the ESS distribution in two differently shaped scaffolds using OCT-based modelling.
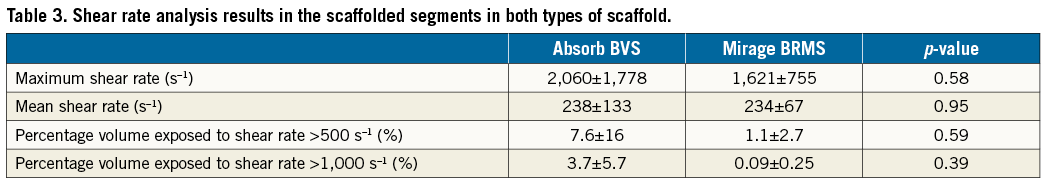
Methods and results: Eight healthy mini pigs were implanted with six Absorb everolimus-eluting bioresorbable vascular scaffolds (Absorb BVS) and five Mirage sirolimus-eluting bioresorbable microfibre scaffolds (Mirage BRMS). Optical coherence tomography (OCT) was performed and strut protrusion was assessed post scaffold implantation. Following the reconstruction of coronary anatomy, blood flow simulation was performed and endothelial shear stress (ESS) was estimated on top of the struts and at luminal surface between the struts in each scaffold. The thicker struts in Absorb (152±140 µm) resulted in an increased protruded distance compared to Mirage (117±123 µm) (p=0.003). This had an effect on the local haemodynamic microenvironment. ESS at the top of the struts was higher in Absorb (1.69±1.20 Pa) than in Mirage (1.53±0.91 Pa) (p<0.001), but lower at inter-strut zones (0.60±0.51 Pa vs. 0.63±0.50 Pa; p<0.01) compared to Mirage. Both scaffold types revealed comparable percentages of vessel luminal surface exposed to recirculation.
Conclusions: Absorb demonstrated higher shear stress on top of the struts compared to Mirage. However, in the inter-strut zones shear stress was higher in Mirage than in Absorb. Further research is required to examine the potential value of in vivo computational modelling in optimising scaffold configuration and clinical outcomes.
Abbreviations
Absorb BVS: Absorb everolimus-eluting bioresorbable vascular scaffold(s)
ESS: endothelial shear stress
Mirage BRMS: Mirage sirolimus-eluting bioresorbable microfibre scaffold(s)
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
PDLLA: poly D, L-lactic acid
PLLA: poly L-lactic acid
Introduction
Local coronary haemodynamic forces, particularly endothelial shear stress (ESS), appear to regulate vessel wall response following implantation of metallic stents or bioresorbable scaffolds (BRS). Numerous in vivo studies implemented intravascular ultrasound (IVUS)-based modelling to examine the association between neointimal hyperplasia and ESS and demonstrated an inverse association between ESS and neointimal growth in bare metal stents1, while in drug-eluting stents this association appears to be regulated by the antiproliferative drug2. Traditionally, coronary reconstruction performed with IVUS has limited resolution and does not allow detailed assessment of lumen morphology, especially in stented segments. Recent reports implementing optical coherence tomography-based modelling have provided further insights into the implications of scaffold implantation on the local haemodynamic microenvironment, enabling for the first time in vivo evaluation of the effect of the protruded struts on the local haemodynamic patterns3,4.
Absorb everolimus-eluting bioresorbable vascular scaffolds (AbsorbTM BVS; Abbott Vascular, Santa Clara, CA, USA) and Mirage sirolimus-eluting bioresorbable microfibre scaffolds (Mirage BRMS; Manli Cardiology, Singapore) have substantial differences in strut thickness, geometrical features and strut connector alignment which, as has been shown in in silico studies, determine flow characteristics and ESS distribution. The aim of this study was to compare the ESS distribution in two differently shaped scaffolds using OCT-based modelling.
Methods
We analysed data from Yucatan mini pigs implanted with Absorb and Mirage scaffolds (Figure 1). Eight Yucatan mini pigs with healthy coronaries underwent percutaneous coronary intervention (PCI) in the three epicardial coronary arteries via the femoral access according to standard procedures5. Six coronary arteries were implanted with a single Absorb and five with a Mirage. The treated coronary arteries were studied by OCT imaging immediately after scaffold implantation. Protocol approval for the animal study was obtained from the Institutional Animal Care and Use Committee and the study was conducted in accordance with the American Heart Association guidelines for preclinical research and the Guide for the Care and Use of Laboratory Animals6.
SCAFFOLD DESIGN
The Absorb BVS is made of poly L-lactic acid (PLLA), coated with a layer of a 1:1 mixture of an amorphous matrix of poly D, L-lactic acid (PDLLA), and elutes everolimus (8.2 μg/mm2). The Absorb is manufactured using extrusion and laser machining techniques, has a strut thickness of 157 µm and is designed with in-phase zig-zag hoops linked with bridges.
The Mirage is made of PDLLA, of which D (dextrorotatory)-isomer constitutes <5% of the total polylactic acid (PLA), is coated with a biodegradable PLA and elutes sirolimus. The Mirage is manufactured by winding the polylactide monofilaments into a helix-coiled structure attached by longitudinal spine microfibres. The struts have a circular shape and a thickness of 125 µm (Figure 1). The helical-coil design is fastened by longitudinal spine microfibres which provide radial strength to the device (148.54 kPa) that is similar to the Absorb (148.00 kPa)7.
DATA ACQUISITION
X-ray angiography was performed using the HiCor cardiac angiography system (Siemens, Erlangen, Germany). OCT was performed following scaffold implantation in all treated coronary arteries. The scaffolded segments were assessed using a C8-XR OCT System (St. Jude Medical, St. Paul, MN, USA) that was pulled back at a speed of 18 mm/sec. A non-occlusive flushing technique was used for blood clearance by injection of contrast media. The acquired data were stored in DICOM format and transferred to a workstation for further analysis.
PROTRUSION ANALYSES
The protrusion analyses on OCT were performed using a special version of QCU-CMS software (version 4.69; Leiden University Medical Center, Leiden, the Netherlands). The analysis on OCT was performed in the scaffolded segment at every 100 µm using a previously presented methodology8 (Figure 1). The details of protrusion analyses can be found in the Supplementary Appendix.
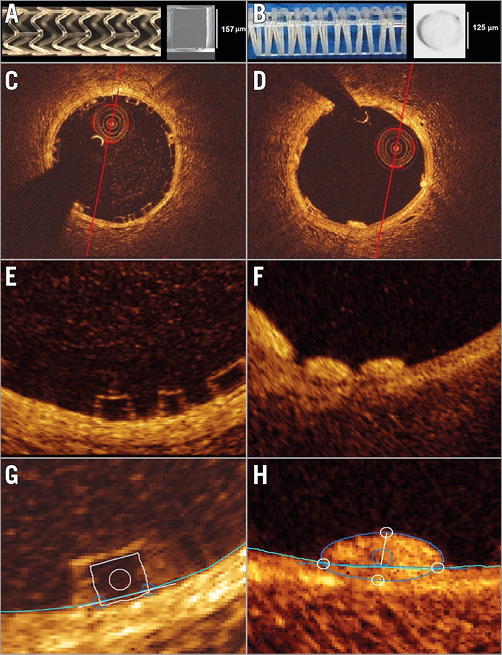
Figure 1. General characteristics of Absorb BVS and Mirage BRMS. Absorb BVS 1.1 and cross-section of an Absorb BVS strut (A). Mirage BRMS and cross-section of a Mirage BRMS strut (B). The strut of the Absorb has a translucent rectangular shape (C, E), whereas the strut of the Mirage is circular, non-translucent (D, F) and protrudes less in the lumen of the vessel. Representation of the protrusion analysis in Absorb (G) and Mirage (H).
CORONARY ARTERY RECONSTRUCTION
Coronary artery reconstruction was performed using a well-established and validated methodology9. Two reconstructions were performed for each scaffold and proximal/distal non-scaffolded segments, one from the flow area borders (flow model) in both scaffolded and non-scaffolded segments, and the other from the flow area in the non-scaffolded segment and the lumen area in the scaffolded segment (reference model) (Figure 2A, Figure 2B). The details of 3D coronary artery reconstruction can be found in the Supplementary Appendix.
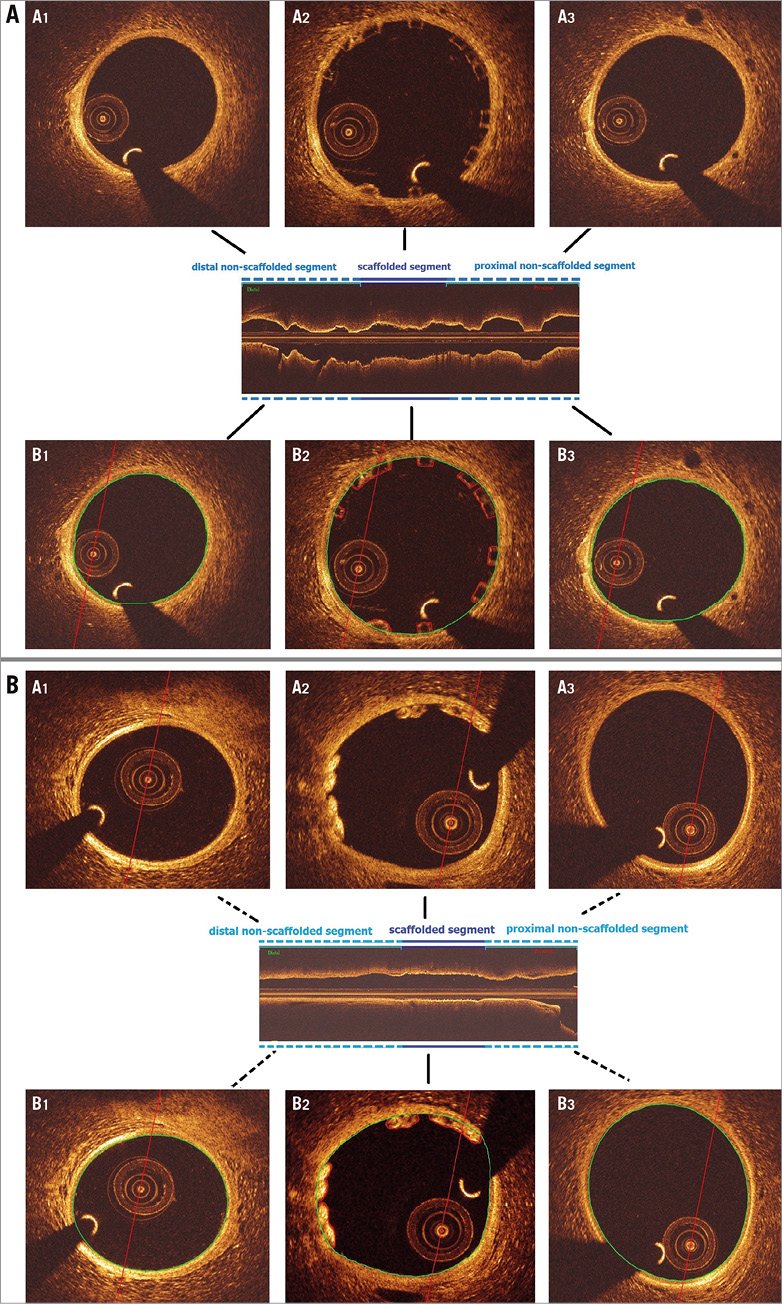
Figure 2. Contour notation methodology in scaffolded and non-scaffolded vessel segments in Absorb BVS (A) and Mirage BRMS (B). In the non-scaffolded and scaffolded segments, the observer delineated the flow area – defined in the non-scaffolded segment by the lumen border (Figure 2A, panels B1 and B3; Figure 2B, panels B1 and B3) and in the scaffolded segment by the adluminal side of the struts and by the lumen border in the areas between the struts (Figure 2A, panel B2; Figure 2B, panel B2).
BLOOD FLOW SIMULATION
The flow models were processed with computational fluid dynamic (CFD) techniques. A finite volume mesh was generated and then blood flow simulation was performed and the ESS was estimated by solving the 3D Navier-Stokes equations (ANSYS® FLUENT®; ANSYS Inc., Canonsburg, PA, USA)10. The ESS was measured at the top of the strut and at the luminal surface between the struts in the scaffolded segment and along the axial direction per 0.2 mm interval with the use of an in-house algorithm (Figure 3). The details of 3D coronary artery reconstruction can be found in the Supplementary Appendix.
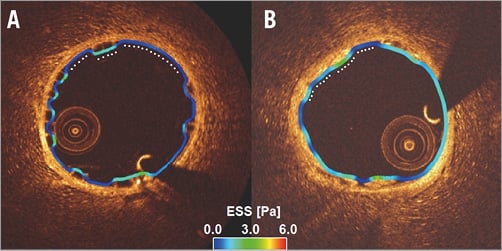
Figure 3. Shear stress quantification methodology. Shear stress is analysed at the top of the struts and in the inter-strut areas separately at each circumferential 1° angle in each cross-section. The white dots depict circumferential 1° angle location. A) Absorb BVS. B) Mirage BRMS.
STATISTICAL ANALYSIS
Continuous variables were tested for normality with the Kolmogorov-Smirnov test and are presented as mean±SD or median (interquartile range) as appropriate. Categorical variables are presented as counts and percentages. Continuous variables were compared by the Kruskal-Wallis test or Mann-Whitney U test. Categorical variables were compared by the Pearson chi-square test. A p-value <0.05 was considered statistically significant.
As the data in the study have multilevel structure and unbalanced design, mixed effects models were used for statistical analysis. To compare the ESS values in different scaffold groups, the multilevel model was initially built with fixed effects on scaffold type, cross-sectional area and interaction of the scaffold type with cross-sectional area and random effects on animal ID, scaffold type and cross-section ID. After comparing different models using maximum likelihood, the best fitted model was selected.
In the protrusion analysis, animal and device types were implemented as random effects, while post-dilatation pressure was input into the model as a fixed effect.
Reproducibility of protrusion analysis for Absorb was previously reported by Sotomi et al8. In the present study, reproducibility of the protrusion analysis for Mirage was assessed with the interclass correlation coefficient (ICC) for absolute agreement (ICCa) with its 95% confidence intervals (CI) using 100 randomly selected Mirage struts8. Analyses were carried out using the statistical analysis programme SPSS, Version 21 (IBM Corp., Armonk, NY, USA), R V. 3.2.3 and the R package lme4 (R Foundation for Statistical Computing, Vienna, Austria)11.
Results
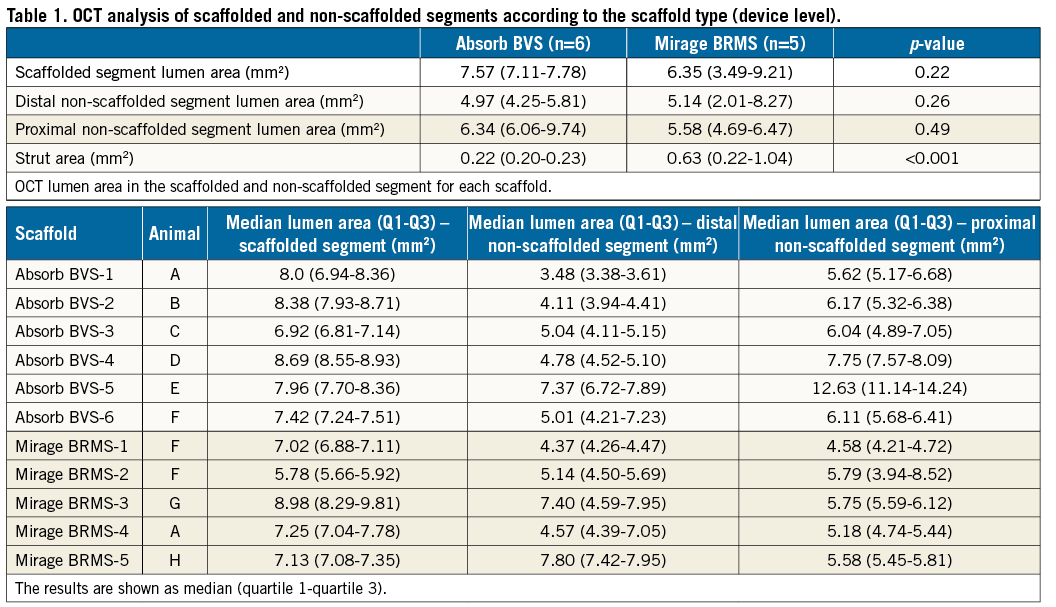
One left anterior descending (LAD) coronary artery, three left circumflex (LCx) and two right coronary arteries (RCA) were implanted with an Absorb, and two LAD, one LCx and two RCA with a Mirage. Scaffold dimensions and procedural parameters are shown in Supplementary Table 1. The dimensions of the proximal edge segment, of the scaffolded segment and of the distal edge segment are shown in Table 1.
PROTRUSION ANALYSIS
The protrusion analyses were performed at cross-section and at device level. In cross-section level analysis, there was a significant difference between the scaffolds in the protrusion distances (152±140 µm for Absorb, 117±123 µm for Mirage; p=0.003), a fact that should be attributed to the different strut thicknesses. The results in the device level analysis were similar. The protrusion distances were significantly increased in the Absorb compared to the Mirage (152±12.2 µm vs. 116±11.1 µm; p<0.0001). The inter-observer reproducibility (ICCa 0.884, CI: 0.827-0.922) and the intra-observer reproducibility (ICCa 0.782, CI: 0.675-0.854) for the protrusion distances indicated good agreement in the Mirage.
ENDOTHELIAL SHEAR STRESS ANALYSIS
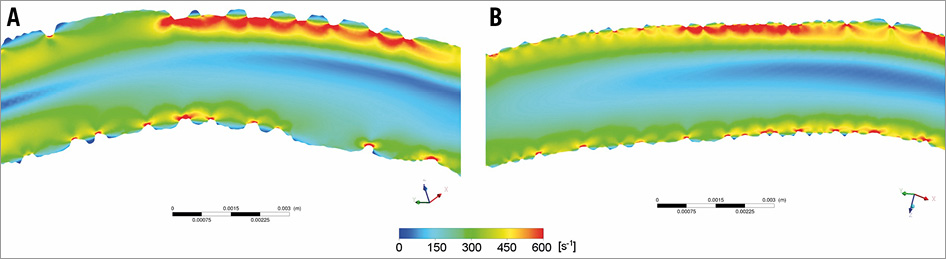
There were several layers of grouping within the data of the study: eight animals (level 3) received scaffold implants in their coronary arteries, and different types of scaffold were used in different vessels within the same animal. Each scaffolded segment had several cross-sections (level 2) and in each cross-section (level 1) the ESS was measured on the top of the struts and at the luminal surface between the struts. After setting the model with random and fixed effects variables, multilevel linear regression analysis revealed that the Absorb had significantly higher ESS at the top of the struts compared to the Mirage. In the luminal surface in the areas between the struts, the Mirage revealed higher ESS compared to the Absorb. On top of the struts the ESS in Absorb was 1.69±1.20 Pa, and 1.53±0.91 Pa in the Mirage (p<0.001), while in the areas between struts the mean ESS values were 0.60±0.51 Pa and 0.63±0.50 Pa, respectively (p<0.001) (Table 2); 52% of the scaffolded surface in the Absorb and 47% in the Mirage (125 µm) was exposed to a low (<1 Pa) athero-promoting ESS environment (p<0.0001) (Figure 4). Lumen cross-sectional area also had an effect on ESS. Increase in the cross-sectional area resulted in a significant decrease in ESS both at the top of the struts and at inter-strut zones (p<0.001 for the ESS on top of the struts, p<0.001 for inter-strut ESS). The maximum and mean shear rates and the percentage volumes of the scaffolded segments exposed to shear rates >500 s–1 and >1,000 s–1 were numerically higher in Absorb than in Mirage; nevertheless, these differences did not reach statistical significance (Table 3, Figure 5). Similarly, the percentage of the recirculation areas was numerically higher in the Absorb, but again this difference did not reach statistical significance (3.26±2.07 vs. 2.71±1.32; p=0.87) (Supplementary Table 2, Figure 6).
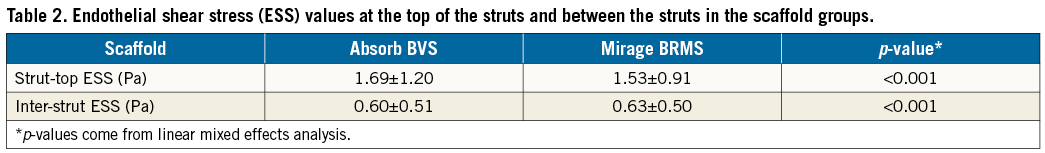
Figure 4. Three-dimensional reconstruction of scaffolded coronary anatomy from the fusion of coronary angiograms and OCT data with the local ESS being portrayed in a colour-coded map (dark blue indicates low ESS <1.0 Pa and aquamarine demonstrates ESS ≥1.0 Pa). A) & C) Absorb. B) & D) Mirage. Note the larger dark blue area in Absorb (C).
 `
`
Figure 5. Increased shear rate at the top of the struts in the Absorb and Mirage scaffolds. A) Absorb. B) Mirage.
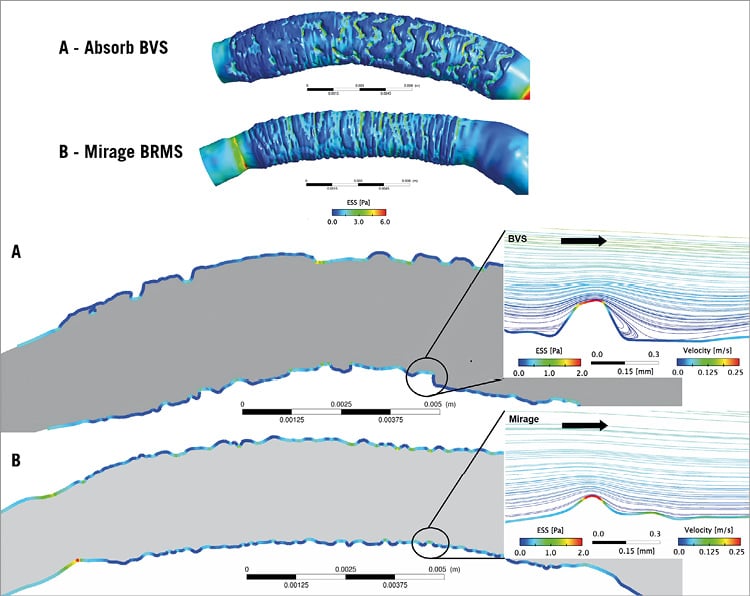
Figure 6. Blood flow streamlines with velocity and ESS colour-coded bars for the Absorb and Mirage. ESS values are high on top of the strut. Low ESS is noted between the Absorb struts (thick rectangular) (A) and between the Mirage struts (thinner circular) (B). While recirculation zones were noted in the distal regions of the Absorb (A), there was no reverse flow in the distal region of the Mirage (B).
Discussion
In this study, we evaluated two different types of BRS with different strut geometries, thicknesses, and strut connector alignment in a porcine coronary artery model. The findings can be summarised as follows. 1) The protrusion distances were higher in Absorb, a fact that should be attributed to the increased strut thickness of this scaffold. This difference resulted in 2) increased ESS at the top of the strut in this device, 3) lower ESS in the areas between the struts compared to the Mirage, 4) numerically higher percentage volumes exposed to high shear rates, and 5) increased recirculation areas in Absorb. These differences, however, did not reach statistical significance for recirculation area and shear rate.
Numerous in silico studies have demonstrated that stent/scaffold design affects the local haemodynamic forces following implantation12,13. The strut shape, strut thickness, maximal coil pitch distance and strut alignment with regard to coronary flow as well as the alignment of the strut connectors appear to determine the local flow patterns13. More importantly, the differences in the local haemodynamic profile following stent/scaffold configuration appear to determine clinical outcomes after PCI. In the bare metal stent era, it was shown that stents with thinner struts were associated with a lower incidence of in-stent restenosis and better clinical outcomes, while in DES the implications of stent configuration on clinical outcomes were suppressed by the antiproliferative drug14-16. In the BRS era, however, there has been a paradigm shift. Recent registry and meta-analysis studies have revealed an increased incidence of stent thrombosis following BRS implantation17,18. These alarming findings have been attributed to the suboptimal local haemodynamics induced by the thick rectangular-shaped struts and scaffold underexpansion; efforts are currently being made to optimise scaffold design.
In silico studies may be helpful in assessing the effect of stent/scaffold design on ESS in treated segments, but they have significant limitations. First, they assume that the stent struts are well apposed. Reports have shown that this is not the case in the clinical setting where up to 30% of the struts are embedded into the vessel wall19. In addition, they assume that the stent/scaffold is fully deployed in the treated vessel and thus they are unable to assess the implications of stent/scaffold underexpansion or early recoil on the ESS distribution20. Finally, they do not take into account the vessel curvature and the changes in vessel geometry induced during device implantation, which depend on vessel curvature and on the mechanical properties of the deployed device, and appear to affect the local haemodynamic forces21.
Taking into account these limitations, we examined for the first time in vivo, in an animal model, the implications of two different scaffold designs with different configuration on the local flow patterns. We found higher ESS on the top of the struts in Absorb compared to the Mirage. Absorb has thicker rectangular-shaped struts which, as has been shown in previous experimental and in silico studies, protrude into the lumen and obstruct flow, resulting in higher ESS at the top of the struts and flow disturbances, recirculation zones, and low ESS in the areas between the struts. On the other hand, the Mirage has a configuration with thinner circular-shaped struts causing lower ESS values at the top of the struts compared to the Absorb scaffold, and causing less flow disruption, resulting in relatively higher ESS in the areas between the struts (Figure 6)15.
In addition to strut geometry, luminal surface coverage ratio and maximal coil pitch distances between the coil rings influence the haemodynamic microenvironment in scaffolded segments. The Mirage (45%) has a significantly higher vessel coverage ratio than the Absorb (27%). The smaller pitch distance (distance between the helical rings) in the Mirage (0.8 mm vs. 1.0 mm in Absorb) is likely to increase flow stagnation areas and decrease the ESS in these segments22,23. However, as was shown in this analysis, strut thickness was the most important determinant of ESS and thus the ESS was higher in the Mirage in the areas between the struts.
Apart from the differences in the ESS, we have noticed differences in the shear rate in the two scaffold designs. Although the mean shear rate values were similar in Absorb and Mirage, the maximum shear rate values and the percentage of the volume of the scaffolded segment exposed to high shear rate were numerically higher in the Absorb compared to the Mirage, indicating a larger variability of the shear rate values in the Absorb. In addition, we found that the recirculation areas were numerically lower in the thinner-strut Mirage (125 µm). This finding is consistent with the results of CFD models which indicate that thinner circular struts lead to a more streamlined flow pattern with less disruption and flow recirculation14. Nevertheless, due to the small sample size of the device level analysis, the difference in shear rate and recirculation areas did not reach statistical significance.
The differences in the haemodynamic profile of Absorb and Mirage may have significant clinical implications. The flow disruption noted in Absorb resulted in low ESS that is associated with increased neointima formation3 and scaffold restenosis, while the flow stagnation and recirculation zones noted in the areas between the struts may promote thrombus formation, as has been shown in histology studies22,24. Finally, high shear rate can trigger activation of platelets and Von Willebrand factor that can promote thrombus formation24. This study demonstrated for the first time that in vivo computational modelling is able to identify and quantify haemodynamic indices which may account for the increased incidence of early scaffold thrombosis and cardiovascular events reported in the early Absorb studies, and thus it can be used to test and improve the haemodynamic profile of future bioresorbable scaffold designs.
Limitations
A significant limitation of the current analysis is that scaffold implantation was performed in healthy coronary arteries. Therefore, it was not possible to examine the implications of scaffold underexpansion20 or the composition of the underlying plaque on strut embedment which potentially influence the local flow haemodynamics. It would be instructive to assess the stent/scaffold behaviour in atherosclerotic vessels to approximate its actual use. Second, the small number of scaffolds studied did not allow us to assess the impact of scaffold design on shear rate and recirculation zones. Moreover, there were differences in the balloon inflation and post-dilatation pressures between groups. Nevertheless, this did not have an impact on strut protrusion, a paradox that should be attributed to the fact that the scaffolds were implanted in healthy vessels. Finally, there were differences in the lumen areas in the two groups which are likely to affect the ESS values. Nevertheless, these differences were not statistically significant. In addition, in the linear mixed effect analysis, scaffold type was independently associated with the ESS distribution.
Conclusions
In vivo computational modelling enables assessment of the effect of different scaffold implantation on local haemodynamic patterns. Mirage with circular, thinner struts, with a helicoidal alignment results in a superior ESS pattern compared to the thick rectangular-shaped struts of the Absorb with a zig-zag hoop alignment. This preclinical study is the preamble of the randomised clinical trial comparing Mirage and Absorb in a first-in-man study. Further research is required to examine the potential value of in vivo computational modelling in optimising scaffold configuration and clinical outcomes.
| Impact on daily practice The Mirage scaffold is a novel BRS and its unique design could provide a better local haemodynamic microenvironment in treated vessels. |
Guest Editor
This paper was guest edited by Lorenz Räber, MD, PhD; Cardiology Department, Bern University Hospital, Bern, Switzerland.
Funding
This study was sponsored by Manli Cardiology, Singapore, Singapore.
Conflict of interest statement
E. Tenekecioglu has received a research grant from TUBITAK (The Scientific Council of Turkey). P. Serruys and Y. Onuma are members of the International Advisory Board of Abbott Vascular. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Methods.
Supplementary Table 1. Inventory of scaffolds and implantation parameters.
Supplementary Table 2. Percentage of recirculation area per scaffolded surface in each scaffold and in the two scaffold groups.
To read the full content of this article, please download the PDF.

