Abstract
Aims: We compared the mechanical and physical properties and the safety from strut fracture of side branch and post-dilatation strategies for the Absorb and DESolve bioresorbable scaffolds with the durable metallic drug-eluting XIENCE Xpedition stent using largely independent bench testing.
Methods and results: The strut thickness and crossing profile of the polymeric scaffolds was greater than those of the metallic drug-eluting stent. While all three devices recoiled after deployment, the DESolve enlarged between 10 mins and one hour returning to the immediate post-deployment diameter (“self-correction”). In 3.0 mm stents/scaffolds, the main branch post-dilatation safe threshold without fracture for Absorb was 3.8 mm at 20 atm, for DESolve was 5.0 mm at 20 atm whereas the ML8 did not fracture. For side branch dilatation with a 3.0 mm non-compliant balloon, the threshold before the Absorb fractured was 10 atm whereas the DESolve and ML8 did not fracture at 22 atm. The safe threshold for mini-kissing balloon post-dilatation in 3.0 mm scaffolds/stents with 3.0 mm non-compliant balloons was 5 atm for the Absorb whereas the DESolve and ML8 did not fracture up to 20 atm.
Conclusions: The metallic stent has thinner struts, lower profile, and greater radial strength than the polymeric scaffolds. Different safe pressure thresholds exist for different scaffolds/stents. Unlike the others, the DESolve showed “self-correction” or enlargement after initial recoil.
Introduction
As early outcomes following percutaneous coronary intervention (PCI) with durable drug-eluting stents (DES) have improved1, attention in interventional cardiology has turned to long-term outcomes in the years after intervention. There is hope that late outcomes after fully bioresorbable scaffold (BRS) implantation will be better because of the absence of a foreign body, restoration of vasomotion, vessel exposure to physiological stresses, positive remodelling, release of the side branch from jail, stabilisation of plaque and ultimately the potential for a normal healed artery2. In addition, there are hypothesis-generating data3 suggesting that chest pain may be less after the Absorb (Abbott Vascular, Santa Clara, CA, USA) BRS implantation than after durable drug-eluting stents (DES). A scaffold is necessary to oppose the negative remodelling component of restenosis and to deliver an antiproliferative drug to counter the intimal hyperplastic component, but beyond about six months a scaffold or stent has no useful function4,5 and may be detrimental. A device that does its job then goes away is an attractive concept.
As the physical properties of various polymers differ from each other and from those of metals, different polymeric BRS behave differently from each other and from metallic DES. Polymeric struts may break more readily than metallic struts6, and the crossing profile of polymeric BRS may be larger than that of metallic DES6. The interventionalist needs to understand the different BRS performance characteristics in order to select appropriate coronary lesions for BRS, and to deliver, deploy and post-dilate BRS appropriately and safely.
This study compares the mechanical and physical properties of the Absorb and DESolve (Elixir Medical Corp., Sunnyvale, CA, USA) BRS with the durable metallic DES, XIENCE Xpedition (bare metal version is MultiLink 8, ML8) (Abbott Vascular), largely using independent bench testing. In particular, we determined safe post-dilatation and side branch dilatation strategies.
Methods
The MultiLink 8 (ML8) stent and its drug-coated counterpart (XIENCE Xpedition) (Figure 1) are constructed from cobalt chromium with in-phase sinusoidal hoops each with six peaks and valleys. There are three connectors per hoop linking peaks and valleys (Figure 1). The manufacturer-determined strut thickness in a radial direction is 89 µm and the width in a circumferential direction is 89-112 µm, with both measurements including the thickness of the durable polymer coating. The manufacturer-calculated potential diameter of a circular cell is 4.2 mm (Figure 1).
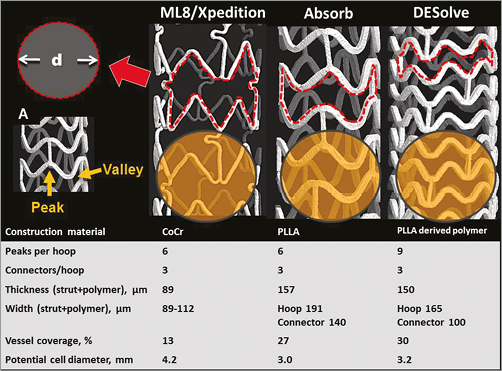
Figure 1. Micro-computed tomography images, design and properties of 3.0 mm examples of the XIENCE Xpedition, Absorb and DESolve stent/scaffolds. The red broken line outlines a cell, and the potential cell diameter (d) is shown. The yellow circle represents a hypothetical side branch ostium with a diameter of 3.0 mm and highlights the potential differences in side branch ostial coverage. In A, a peak and a valley of a hoop are indicated.
The Absorb scaffold (Figure 1) constructed from poly-L-lactic acid has in-phase sinusoidal hoops with six peaks and valleys per hoop and three straight longitudinal connectors linking peaks and valleys of adjacent hoops. The manufacturer-measured thickness (radial direction) of all hoops and connectors is 157 μm. The connector width (140 μm) is less than the hoop width (191 μm). These measurements include the resorbable coating. The manufacturer-calculated potential circular diameter of a cell is 3.0 mm for the 2.5/3.0 mm scaffold.
The DESolve scaffold is constructed from a poly-L-lactic-acid-based material. The 3 mm device has sinusoidal hoops with nine peaks and valleys linked by three straight connectors except at the ends where the peaks and valleys are out-of-phase and all peaks and valleys are linked (Figure 1). The manufacturer-determined hoop and connector thickness including the polymer coating is 150 µm. The width in a circumferential direction is 165 µm for hoops and 100 µm for connectors (Figure 1).
The manufacturers report that the percentage of vessel wall covered by a 3.0 mm stent/scaffold deployed at nominal pressure is 13%, 27% and 30% for Xpedition, Absorb and DESolve, respectively (Figure 1).
The coatings of the XIENCE Xpedition and of the Absorb scaffold contain and control the release of the antiproliferative drug everolimus (Novartis, Basel, Switzerland). The DESolve scaffold contains and controls the release of novolimus (Elixir Medical Corp.).
DEPLOYMENT AND IMAGING TECHNIQUE
All scaffold deployments were in a water bath at 37°C. Photographs were with an EOS-1D Canon digital camera (Canon, Inc., Tokyo, Japan) with a Leica Z6 APO microscopic lens (Leica Microsystems, Wetzlar, Germany). Measurements were made from photographs using Image Pro Plus Software (Version 7.0.0.591; Media Cybernetics, Inc., Bethesda, MD, USA). Calibration was checked by photographing a graticule of known dimensions. Balloon dilatations were done with non-compliant (NC) balloons. One scaffold was used for sequential tests, and different sites on a long scaffold were used to reduce the number of scaffolds needed. After removal from the water bath for inspection, scaffolds were re-acclimatised in the water bath for at least one minute before further post-dilatation or side branch dilations. Once a scaffold was unpacked, the expansion, post-dilation, and/or side branch dilation sequences were completed in a timely fashion.
In addition, some scaffolds were imaged using micro-computed tomography (micro-CT) (SkyScan 1172; SkyScan, Kontich, Belgium). These micro-CT images could be manipulated electronically allowing image rotation, electronic dissection, and “fly through” so that stents could be examined from different perspectives and potentially confusing overlying struts removed.
CROSSING PROFILE
Crossing profiles (the diameter of an undeployed scaffold mounted on its delivery system) (n=5) were photographed in air, rotated 90° then photographed again for measurements.
RECOIL
Recoil was defined as the percentage scaffold or stent diameter change from that measured on the inflated balloon at nominal pressure to that after balloon deflation. Scaffolds (n=5) of each design were expanded unconstrained in a water bath at 37°C at nominal pressure and photographed. After balloon deflation, the scaffolds and stents were then photographed at one, 10 and 60 minutes. Diameter measurements were made from photographs at each time point.
RADIAL STRENGTH
The radial strength of an expanded stent/scaffold is the ability to resist compressive forces. We made cross-sectional area measurements of expanded ML8 stents (n=5) and Absorb scaffolds (n=5) with intravascular ultrasound during incremental increases in external pressure in a custom-made water bath. For the comparison of DESolve and Absorb scaffolds, Elixir Medical used an iris-like radial compression fixture attached to a calibrated tensile-compression force test stand that applied a constant reduction in diameter while measuring force. The pressures needed to reduce the device cross-sectional area by 25% were assessed.
MAIN BRANCH POST-DILATATION AND STRUT FRACTURE
Scaffolds, 3.0 mm in diameter, were deployed unconstrained in the water bath at 37ºC then post-dilated with increasing NC balloon size up to 20 atm pressure or until a strut fracture was observed. The percentage of scaffolds with fracture was plotted against balloon diameter.
SIDE BRANCH DILATATION
To determine the effect of side branch dilatation, 3.0 mm stents/scaffolds were deployed in the water bath in silicone phantoms with “B” angle7 between the main branch and side branch of 30°. The main branch phantom diameter was 3.5 mm tapering to 3.0 mm distal to the side branch origin, and the side branch diameter was 3.0 mm. Side branches were dilated with NC balloons of increasing diameters. The stents/scaffolds were observed for strut fracture and imaged by fluoroscopy and photography. In addition to the durability of struts, we documented scaffold distortion after side branch dilatation then correction of distortion with mini-kissing balloon post-dilatation (mini-KBPD)8.
ASSESSMENT OF THE RELATIONSHIP BETWEEN MINI-KISSING BALLOON POST-DILATATION PRESSURE AND STRUT FRACTURE
With mini-KBPD, the proximal markers of the balloons are not aligned but offset so that the proximal main branch is exposed to the inflation of only one balloon and the bifurcation to the inflation of both balloons8. After 3.0 mm diameter scaffolds were deployed in a phantom with a 30° side branch angle, two 3.0 mm NC balloons in a mini-KBPD configuration were inflated slowly and simultaneously up to 20 atm, or until strut fracture was observed.
STATISTICS
Descriptive statistics of the data are provided as mean±SD. The difference in crossing profile, recoil and radial strength between stents/scaffolds was compared with either the Mann-Whitney U test or the Wilcoxon signed-rank test. Statistical analyses were performed using SAS statistical software, version 9.3 (SAS Institute, Cary, NC, USA). All p-values resulted from two-sided tests and a p-value of <0.05 was considered statistically significant.
Results
All tests were with 3.0 mm nominal diameter stents/scaffolds.
CROSSING PROFILE
The crossing profile (n=5 for each) of the Xpedition (1.14±0.01 mm) was less than that of the polymeric scaffolds (Absorb [1.43±0.02 mm] and DESolve [1.44±0.02 mm], p=0.04 and p=0.05, respectively).
RECOIL
The external diameters of the Absorb and DESolve scaffolds at deployment were greater at baseline than that of the ML8 stent because of their thicker struts (Figure 2). By one minute after deployment, all three devices had recoiled by approximately 0.1 mm. Between one minute and 10 minutes, there was not much change in diameter. Between 10 minutes and one hour, the Absorb scaffolds and ML8 stents showed little change, but the DESolve scaffold increased in diameter to baseline dimensions (“self-correction”), so that its diameter (3.52±0.14 mm) was larger than that of the Absorb scaffold (3.30±0.04 mm) (Figure 2).
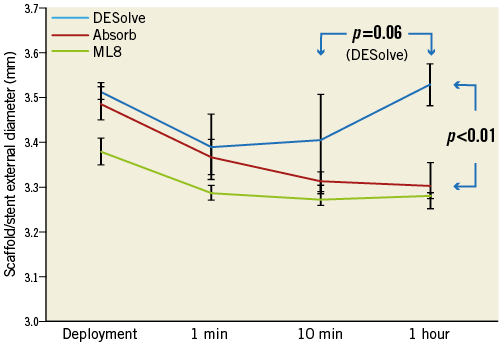
Figure 2. Recoil. Scaffold (or stent) external diameter immediately after deployment, at one minute, 10 minutes, then at one hour.
RADIAL STRENGTH
The ML8/Xpedition stents had greater radial strength than the Absorb scaffold which had greater radial strength than the DESolve scaffold (Table 1).

MAIN BRANCH POST-DILATATION
The ML8 stent did not fracture with main branch post-dilatation with balloon diameters up to 5.5 mm at 20 atm pressure (Figure 3). The DESolve did not fracture at diameters of 5.0 mm or less and the Absorb did not fracture at diameters of 3.8 mm or less at 20 atm pressure. With increasing pressure, the sinusoidal hoops of the DESolve straightened and stretched before fracturing (Figure 4). The Absorb hoops also straightened and may not have stretched as much before fracturing.
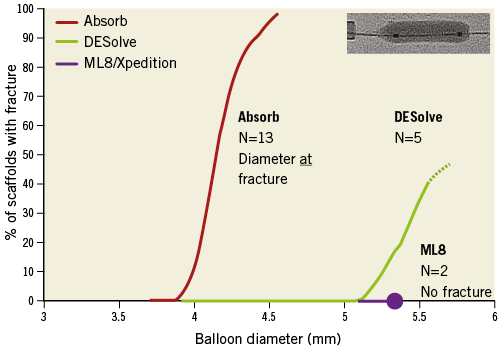
Figure 3. Main branch post-dilatation balloon diameter and percentage of 3.0 mm diameter devices with strut fracture.
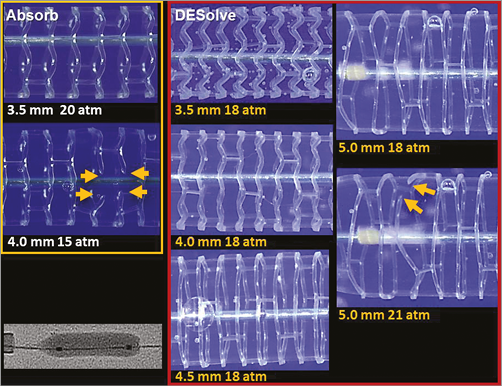
Figure 4. Photographs (not to scale) of the Absorb and DESolve scaffolds with increasing main branch post-dilatation balloon diameter (mm) at pressures as indicated. The yellow arrows indicate strut fractures which were caused for these individual scaffolds by a balloon diameter of 4.0 mm at 15 atm for the Absorb scaffold, and of 5.0 mm at 21 atm for the DESolve scaffold.
SIDE BRANCH DILATATION AND SCAFFOLD FRACTURE
There were no fractures observed in the DESolve scaffold or ML8 stent (Figure 5) even with side branch dilatation with a 3.0 mm NC balloon at 22 atm. With the Absorb scaffold there were no fractures at inflation pressures up to and including 10 atm (Figure 5). Dilatation through the side of scaffolds or stents caused distortion best corrected by kissing balloon post-dilatation or mini-KBPD.
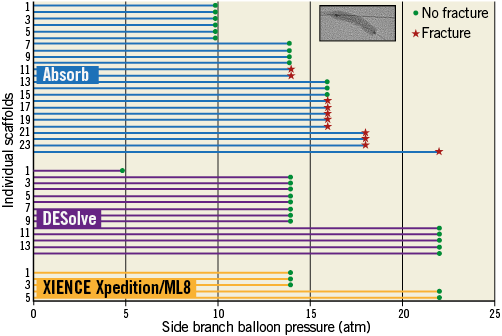
Figure 5. Side branch dilatation in a 3.0 mm scaffold with increasing 3.0 mm non-compliant balloon pressure for individual ML8 stents and Absorb and DESolve scaffolds. The green point represents a scaffold inspection that revealed no strut fracture. The red star represents inspection with strut fracture(s) observed. There were no fractures observed in DESolve scaffolds or ML8 stents even with side branch dilatation at 22 atm. With the Absorb scaffold there were no fractures at 10 atm pressure or less.
MINI-KISSING BALLOON POST-DILATATION
With mini-KBPD with two 3.0 mm NC in 3.0 mm scaffolds/stents, there were no strut fractures in the DESolve scaffold or ML8 stent up to 20 atm pressure (Figure 6). With the Absorb scaffold there were no fractures at 5 atm or less (Figure 6).
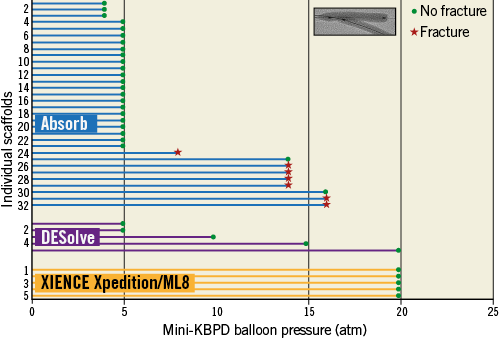
Figure 6. Mini-kissing balloon post-dilatation in a 3.0 mm scaffold with two 3.0 mm non-compliant balloons with pressure for Absorb and DESolve scaffolds. The insert in the top right corner shows balloons inflated using the mini-KBPD strategy where the proximal main branch is exposed to the diameter of only one balloon and the bifurcation to the diameters of both balloons. A green point represents a scaffold inspection with no strut fracture. The red star represents inspection with strut fracture(s) observed. There were no strut fractures in the ML8 stent or DESolve scaffold even at 20 atm pressure with mini-KBPD. With the Absorb there were no fractures at 5 atm or less, so this is a safe threshold for mini-KBPD in a 3.0 mm Absorb scaffold with two 3.0 mm non-compliant balloons.
Discussion
The main findings of this study are for 3.0 mm scaffolds/stents:
– The main branch post-dilatation safe threshold without fracture was 3.8 mm at 20 atm for the Absorb scaffold, and 5.0 mm at 20 atm for the DESolve scaffold, while the ML8 stent did not fracture (Figure 3, Figure 4).
– For side branch dilatation with a 3.0 mm NC balloon, the safe pressure threshold without fracture for the Absorb scaffold was 10 atm. There were no fractures of the DESolve scaffold or the ML8 stent up to 22 atm (Figure 4, Figure 5).
– The safe threshold for mini-KBPD with two 3.0 mm NC balloons was 5 atm for the Absorb scaffold while the DESolve scaffold and the ML8 stent did not fracture up to 20 atm (Figure 6). The strut fracture potential with different post-dilatation strategies is summarised in Figure 7.
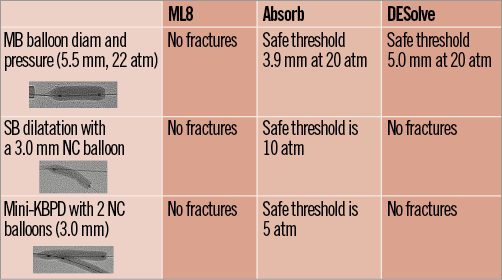
Figure 7. Summary of post-dilatation strategies and safe thresholds for Xpedition/ML8, Absorb and DESolve.
– While all three device designs recoiled after deployment, the DESolve scaffold differed when, between 10 minutes and one hour, it enlarged (“self-correction”) to return to the immediate post-deployment diameter (Figure 2).
– The crossing profile of the Xpedition stent (1.14±0.01 mm) was less than that of the polymeric scaffolds (Absorb [1.43±0.02 mm] and DESolve [1.44±0.02 mm]), p=0.04 and p=0.05, respectively.
Concern about potential Absorb scaffold fracture6 has altered deployment protocols and interventionalists’ behaviour. To ensure scaffolds deployed are of appropriate diameter for an artery and to limit the risk of post-dilatation-induced fracture, the protocol for the Absorb II trial comparing patients randomised to receive the Absorb scaffold or the XIENCE metallic DES3 mandated on-line quantitative angiographic assessment of target vessel diameter. In addition, to limit the risk of fracture, the protocol recommended against post-dilatation of the Absorb scaffold. However, if post-dilatation were necessary, it recommended using a non-compliant balloon not more than 0.25 mm larger than the scaffold nominal diameter. As a consequence, smaller balloons and lower pressures were used for post-dilatation of Absorb scaffolds than of XIENCE stents3. An additional consequence was that the final procedural minimum luminal diameter was smaller for Absorb than for XIENCE (2.22±0.30 mm and 2.50±0.03 mm, respectively, p<0.001). The difference in final procedural luminal size was not due to differences in immediate recoil, as the mean recoil in both devices was 0.19 mm. While differences in final procedural size might influence outcomes after metallic stenting9, after bioresorbable scaffold implantation other factors such as positive remodelling operate10. The risk of strut fracture is low with the DESolve scaffold (Figure 3), so that there may be greater confidence in the safety of post-dilatation. Consequently, final procedural luminal diameter might be similar to metallic DES if radial strength is adequate.
There are reports of possible increased scaffold thrombosis after Absorb scaffold implantation11. If this increase is real, it may be due to scaffold malapposition related to inadequate post-dilatation driven by the fear of strut fracture. Better understanding of safe post-dilatation may lead to improved deployment.
Because of the risk of scaffold fracture, we recommend8 that, when treating a bifurcation with an Absorb using a provisional side branch strategy, the scaffold should be sized to the proximal vessel (in contrast to the European Bifurcation Club recommendation for metallic stents12,13) to reduce the risk of proximal strut fracture with post-dilatation. We then recommend proximal optimisation followed if necessary by side branch dilatation then mini-KBPD to correct deformation8. The DESolve and of course the Xpedition/ML8 can be deployed in the same manner as metallic stents because of low or absent risk of strut fracture.
The DESolve scaffold recoil in the first minute after deployment is similar to that of the Xpedition stent and the Absorb scaffold. However, unlike these it subsequently undergoes expansion (“self-correction”), so that by one hour it achieves a similar diameter to that at original deployment (Figure 2). It is expected that this property will correct for minor malapposition14. Whether this is an advantageous feature, for instance in acute myocardial infarction or after chronic total occlusion treatment when stents may be undersized, is unknown. The differences in recoil over time between scaffolds raises issues about when recoil should be measured clinically. If recoil is measured immediately after device deployment, results may be very different from measurements made at one hour (Figure 2). The role of recoil in clinical outcomes after scaffold implantation is unknown.
The ML8/Xpedition has greater radial strength than the bioresorbable scaffolds and the Absorb has greater radial strength than the DESolve (Table 1). Whether these numerically small differences are clinically important is unknown. It may be that stents or scaffolds with greater radial strength and less recoil will have better initial angiographic outcomes than those with less radial strength and/or more recoil. This could argue particularly for meticulous predilatation before scaffolding with a device with less radial strength and/or more recoil. The relationship of immediate angiographic outcomes with scaffolds to long-term outcomes is complex because of positive remodelling as scaffolds resorb. The thickness of struts including the drug coating for current iterations of Absorb (157 µm) and DESolve (150 µm) is greater than for the Xpedition/ML8 stent (89 µm). Thick struts compared with thinner struts are disadvantageous because they increase the crossing profile, delay endothelialisaton, and alter local blood flow with adverse effects on shear stress and potential thrombosis. Both manufacturers of the resorbable scaffolds are working on iterations with thinner struts in the order of 100 µm and that from Elixir already has CE mark approval.
Strut thickness, and perhaps more importantly strut width, may influence periprocedural myocardial necrosis. In the TAXUS ATLAS trial15, the thicker strut TAXUS Express stents caused more periprocedural myocardial necrosis than the thinner strut TAXUS Liberté stents that were made from the same metal and eluted the same drug from the same polymer. The polymeric scaffolds in the current study have thicker and wider struts than the XIENCE Xpedition (Figure 1). Surprisingly, in the Absorb II trial which randomised patients to receive a XIENCE (same strut thickness as XIENCE Xpedition) or an Absorb device, cardiac biomarker rise within 48 hours after the index procedure and per-protocol periprocedural myocardial infarction did not differ between arms3. However, the Absorb II trial excluded patients with side branches of 2.0 mm or more if they were to be covered by a stent or scaffold.
The crossing profiles of the polymeric scaffolds are larger than those of the ML8/Xpedition so that these scaffolds are more difficult to deliver, including through the side of a scaffold16. The next-generation Absorb and DESolve scaffolds with thinner struts will have lower crossing profiles. While strut thickness is a major contributor to crossing profile, delivery balloon technology and the crimping process are also probably key contributors.
The arterial coverage by struts is greater for the DESolve (30%) and the Absorb (27%) than for the ML8/Xpedition (13%). Potential side branch ostial coverage is shown in yellow in Figure 1. Serial OCT studies have shown that bridging between Absorb struts reduces the side branch ostial flow area over time before the struts are resorbed17,18. With increased ostial coverage, such as with DESolve and Absorb, and with the strut thickening and bridging, there may be greater reduction in ostial flow area than with devices with lesser ostial coverage by struts. It may be that side branch dilatation and mini-KBPD will partially clear struts from the SB ostium, hence reducing the potential for reduced ostial flow area due to strut thickening.
Limitations
Bench testing does not always predict clinical performance of devices. We studied a limited number of scaffolds/stents. Results apply to specific bioresorbable scaffolds and do not predict behaviour of other bioresorbable scaffolds which may have profoundly different performance characteristics. We have measured radial strength only after deployment at nominal pressure. It is likely that, while the DESolve can be post-dilated beyond 4.0 mm, the radial strength may be less than that of the ML8/Xpedition at a similar diameter. The radial strength measurement for DESolve was carried out by the manufacturer. However, the radial strength of the Absorb from Elixir is almost identical with our measurement (Table 1).
Summary
We provide preclinical bench testing data for the two commercially available bioresorbable scaffolds which we compared with a state-of-the-art metallic DES. Various key product performance properties, such as crossing profile, recoil, post-dilatation overexpansion, main branch and side branch dilatation and radial strength of the scaffold and stent, have been presented. As physicians start to increase the use of BRS in daily practice, such independent testing is critical for a better understanding and use of these products in the most effective and safe manner.
With main branch post-dilatation of the Absorb and DESolve scaffolds, there are pressure thresholds below which strut fracture does not occur. The threshold for DESolve was higher than that for Absorb. There are fracture thresholds for mini-KBPD with the Absorb scaffold while no fractures occurred with DESolve or ML8/Xpedition. Recoil varies over time, with the DESolve showing expansion by one hour, so that the timing of recoil measurement in clinical trials influences results. The metallic durable DES has thinner struts, lower crossing profile, and greater radial strength than the polymeric bioresorbable scaffolds. DESolve scaffolds with thinner struts (100 µm) are in clinical trials. Absorb scaffolds with thinner struts are under development.
| Impact on daily practice While bioresorbable scaffolds are promising because of potential late advantages after implantation, the interventional cardiologist needs to understand the differences from metallic stents for different scaffold designs to ensure safe and effective delivery and adequate deployment. Current-generation polymeric scaffolds differ from metallic stents in that struts may be more easily fractured and the crossing profile is larger. Post-dilatation, side branch dilatation and mini-kissing balloon post-dilatation can all be performed safely without strut fracture if safe pressures and balloon sizes are selected according to our testing results. Different scaffold designs may have profoundly different performance characteristics. |
Funding
Auckland Heart Group Charitable Trust and Elixir Medical. Some scaffolds provided by Abbott Vascular.
Conflict of interest statement
J. Ormiston is an advisory board member for Abbott Vascular and Boston Scientific, and has received minor honoraria from them. O. Darremont is an advisory board member for Abbott Vascular. The other authors have no conflicts of interest to declare.

