
Abstract
Aims: The aim of the study was to compare retrospectively the acute mechanical performance of the Absorb vs. DESolve scaffolds in terms of appropriate deployment with OCT.
Methods and results: Final post-deployment OCT pullbacks of consecutive patients treated with either Absorb or DESolve were reviewed. The following parameters were calculated and compared: mean and minimal lumen area (MLA), residual in-scaffold area stenosis (RAS), incomplete strut apposition (ISA), tissue prolapse area, eccentricity index, asymmetry index, strut fracture and edge dissection. A total of 72 patients were included. The Absorb group consisted of 35 patients treated with 63 Absorb scaffolds and was compared to a well-matched group of 37 patients treated with 50 DESolve scaffolds. Baseline characteristics did not differ significantly between the two groups. Procedural characteristics were different with respect to maximal balloon inflation pressure (Absorb vs. DESolve: 21.5±0.4 atm vs. 16.8±3.8 atm, p<0.01) and mean NC balloon diameter used for post-dilatation (Absorb vs. DESolve 3.3±0.4 mm vs. 3.5±0.4 mm, p<0.01). OCT analysis showed similar MLA (Absorb vs. DESolve: 5.8±1.9 mm2 vs. 6.1±2.6 mm2, p=0.43) and mean luminal area (Absorb vs. DESolve: 7.1±2.2 mm2 vs. 7.2±1.9 mm2, p=0.77). The mean eccentricity index was 0.85±0.05 with Absorb and 0.80±0.05 with DESolve, p<0.01. There was no difference in the incidence of overall ISA. A smaller prolapse area was found with Absorb (Absorb vs. DESolve 1.0±1.1 mm2 vs. 3.6±6.2 mm², p<0.01).
Conclusions: The two scaffolds showed similar MLA while there was a trend towards a lower RAS and a larger maximum and minimum scaffold diameter with DESolve. The DESolve scaffold was more eccentric as compared to the Absorb. These results might be related to the DESolve’s unique expansion properties or they may reflect baseline and procedural differences which cannot be excluded in a retrospective study. Randomised studies are needed to address this aspect.
Abbreviations
BRS: bioresorbable scaffold
ISA: incomplete strut apposition
MLA: minimal lumen area
NC: non-compliant
OCT: optical coherence tomography
PLLA: poly-L-lactic acid
QCA: quantitative coronary angiography
RAS: residual area stenosis
RVA: reference vessel area
Introduction
Bioresorbable scaffolds (BRS) have emerged as a potential major breakthrough for the treatment of coronary artery lesions1-3. The most frequently used polymer in the current generation of BRS is poly-L-lactic acid (PLLA), a polymer already in widespread use (with applications such as resorbable sutures, soft-tissue implants, orthopaedic implants, and dialysis media) with all the key mechanical properties to be the candidate material for coronary indications4.
Whereas several PLLA BRS have been developed and tested5, only a few of these have reached the stage of first-in-man human implantation, and even fewer have been approved for clinical use, as mechanical and biological equivalence between different platforms cannot be inferred but has to be rigorously proved.
Currently, in the European Union, two BRS, namely the Absorb (Abbott Vascular, Santa Clara, CA, USA) and the DESolve® (Elixir Medical Corporation, Sunnyvale, CA, USA) have obtained approval for clinical use (Figure 1). Interestingly, whereas a growing body of evidence supports the safety and efficacy of the Absorb6-8, few data are available for the recently approved DESolve scaffold9,10.

Figure 1. Schematic representation of the two scaffolds. A) Absorb; B) DESolve. Both the scaffold platforms are PLLA-based. They have a similar crossing profile with struts as thick as 150 mcm. The resorption should be shorter with DESolve (about two years) as compared with Absorb (more than three years). The Absorb is coated with a non-erodible polymer loaded with 100 pg/cm² of everolimus. The DESolve is coated with approximately 5 mcg of novolimus per mm of scaffold length (e.g., 65 mcg for a 14 mm scaffold and 85 mcg for an 18 mm scaffold) delivered via a proprietary bioresorbable PLLA-based polymer, which allows sustained release of the majority of the drug in approximately four weeks.
The aim of this study was to compare the acute mechanical performance of the DESolve versus the Absorb scaffold in two retrospective cohorts of all-comers patients (and lesions), in whom OCT was used to guide the procedure and assess appropriate scaffold deployment11,12.
Methods
STUDY POPULATION
In this retrospective study, consecutive patients undergoing PCI with either an Absorb or a DESolve scaffold under OCT guidance and with a final pullback (i.e., after the last post-dilation) were enrolled. The procedures were performed at three centres: the Royal Brompton Hospital, London, UK, the Careggi Hospital, Florence, Italy, and the University Hospital of Giessen, Medizinische Klinik I, Giessen, Germany. The DESolve group included patients treated with DESolve from May to November 2014, while the Absorb group included patients treated from September 2012 to August 2013. All patients signed an informed consent for PCI with scaffold deployment and OCT guidance.
QCA ANALYSIS AND LESION CHARACTERISATION
QCA was performed offline using the CAAS QCA-2D system (Pie Medical Imaging BV, Maastricht, The Netherlands). For each lesion, minimum lumen diameter (MLD), reference vessel diameter (RVD) through automatic interpolation, percentage area stenosis (%AS) and lesion obstruction length (LL) were measured. Data from QCA were not used for the selection of scaffold dimensions during the interventions.
The largest balloon diameter and maximal inflation pressure during lesion predilatation were recorded and used to calculate the balloon/artery ratio.
TREATMENT PROCEDURES
Vessel sizing, lesion preparation, scaffold deployment and post-deployment optimisation were performed according to our common practice12.
Lesions were treated with predilatation using conventional semi-compliant or NC balloons. The use of additional devices (cutting balloons or rotablator) was left to the operator’s discretion.
The deployment of BRS was performed using slow balloon inflation (2 atm every five seconds keeping the delivery balloon inflated for at least 30 seconds if possible) without exceeding the rated pressure indicated in the product instructions for use (16 atm for both the Absorb and DESolve). Post-dilatation with OPN NC™ balloons (SIS Medical AG, Winterthur, Switzerland) was allowed when pressures higher than 30 atm were required13 and was left to the operator’s discretion. OCT was used for procedural guidance and a final pullback was performed and used for the study analysis.
OCT ACQUISITION
Frequency domain OCT was performed using the ILUMIEN™ OPTIS™ system (St. Jude Medical, St. Paul, MN, USA). The Dragonfly Duo imaging catheter (St. Jude Medical) was used. Automatic pullbacks were performed at 36 mm/s during contrast injection at a rate of 3-5 ml/s using a power injector. The OCT catheter was inserted distally to the treated segment and the pullback continued until either the guiding catheter was reached or the maximal pullback length (75 mm) was completed.
OCT OFF-LINE ANALYSIS
The OCT measurements were repeated off-line using the LightLab Imaging workstation (St. Jude Medical). The analysis of contiguous cross-sections was performed at 1 mm longitudinal intervals within the entire scaffolded segment and at 5 mm intervals proximal and distal to the scaffold in order to measure the proximal and distal reference vessel area (RVA) and to identify dissections. RVA was calculated as the mean of the two largest luminal areas in the 5 mm proximal and distal to the BRS edge14. In case of absence of a meaningful proximal or distal segment due to the ostial location of the lesion or the presence of a large side branch at the scaffold edge, only a proximal or distal reference cross-section was used to calculate RVA. Scaffold edge dissection was defined as a disruption of the vessel luminal surface at the scaffold edge with visible flap. Scaffold fracture was suspected in the presence of isolated struts lying grossly unapposed in the lumen or in the presence of one strut on top of the other. For each cross-section analysed, the area, mean, maximal and minimal diameter of the scaffold were manually contoured and measured.
Incomplete strut apposition (ISA) was defined as the presence of struts separated from the underlying vessel wall14. Tissue prolapse was defined as the presence of tissue protruding between scaffold struts extending into the lumen as a circular arc connecting adjacent struts. Examples are illustrated in Figure 2 and Figure 3.
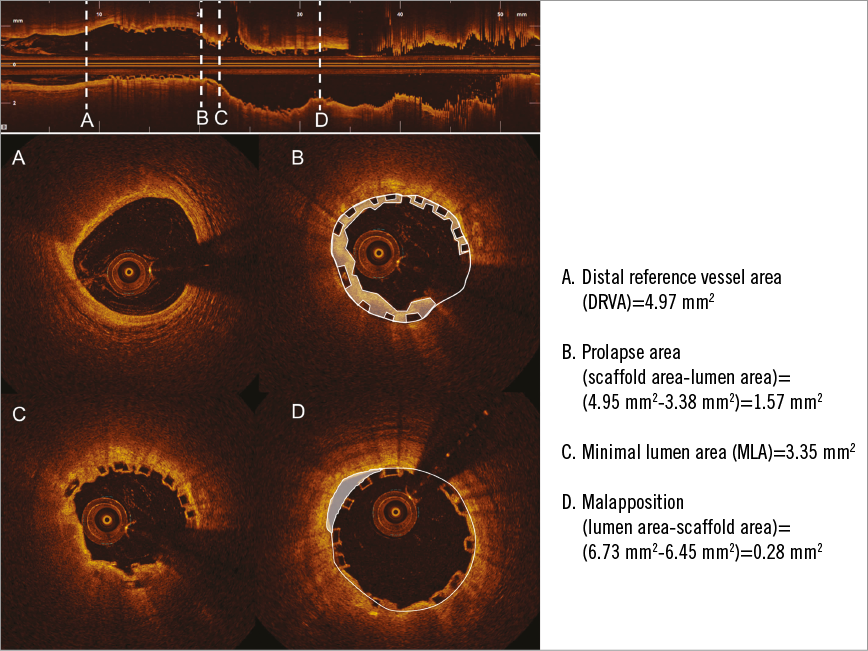
Figure 2. Example of evaluation of OCT parameters used to assess the appropriate deployment of an Absorb scaffold implanted into the left anterior descending artery (segment 7). A) The distal RVA. B) Tissue prolapse. The prolapse area is highlighted in white and was calculated as the difference between the scaffold and lumen area. C) Cross-section of the MLA of the scaffold. D) An example of ISA. There are two malapposed struts between nine and 12 o’clock. The malappostion area is highlighted.
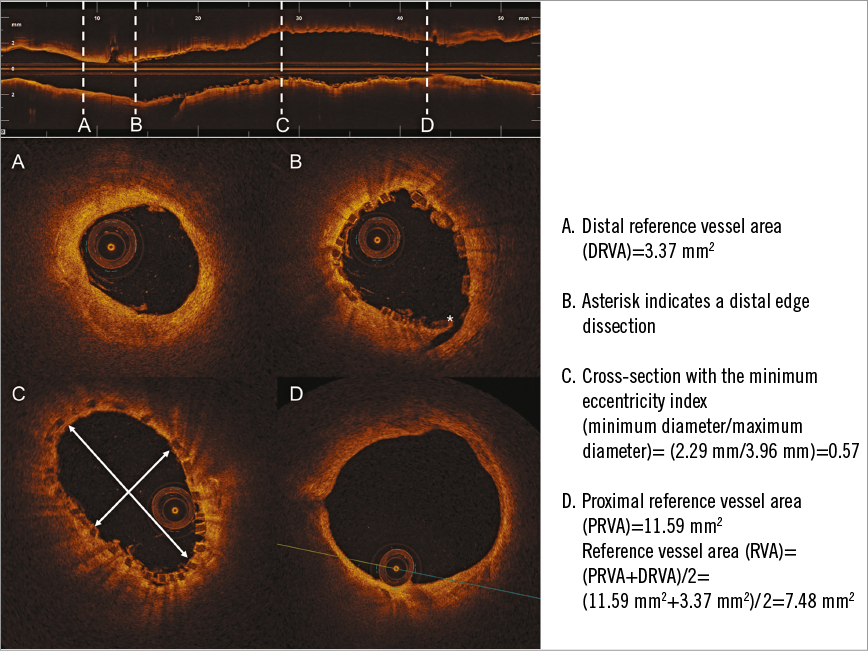
Figure 3. An example of OCT assessment of correct DESolve scaffold deployment into the right circumflex artery. The RVA was computed by dividing the sum of the distal (A) and proximal (D) reference vessel area. B) An edge dissection (asterisk). C) The scaffold cross-section with the minimum eccentricity index.
The following quantitative parameters were calculated for each scaffold, as previously reported14:
Percentage of ISA struts: calculated as the percentage of the total number of malapposed struts observed at 1 mm intervals/total number of struts observed at 1 mm intervals.
The presence of malapposition at the proximal and distal ends of the scaffolds was specifically assessed. Proximal and distal ends were defined as the last 5 mm of scaffold within the scaffold proximal and distal edges. A scaffold was counted as scaffold with malapposition at the ends in case of malapposed struts at the proximal and/or distal ends.
ISA area, mm2.
Tissue prolapse area (mm2): calculated as the difference between the abluminal scaffold area and the lumen area.
Percentage of in-scaffold residual area stenosis (RAS) calculated as (1-[min lumen area/RVA])×100.
Eccentricity index: ratio between the minimal and the maximal diameter. For each scaffold the eccentricity index was computed at each cross-section analysed (i.e., each 1 mm interval within the scaffold) and both the minimal and the mean eccentricity index were reported.
Asymmetry index: defined as (maximum scaffold diameter–minimum scaffold diameter)/(maximum scaffold diameter).
FOLLOW-UP
Clinical follow-up was obtained at one and six months by direct clinical examination according to institutional protocols and is reported only for descriptive purposes.
STATISTICAL ANALYSIS
Descriptive statistics (means and standard deviations for continuous variables with normal distribution, counts [%] for categorical variables) were computed according to treatment type (Absorb vs. DESolve). Comparison between groups for continuous variables was performed by unpaired t-test (in case of parametric distribution) or Mann-Whitney U test (in case of non-parametric distribution), as appropriate. Univariate associations between treatment type and coronary lesion features were examined using two-way contingency tables. For categorical variables, significance of associations was assessed using the chi-squared test or the Fisher’s exact test, as appropriate. For all the statistical tests used, a p-value of <0.05 was required to reject the null hypothesis. The statistical analysis was performed using the SPSS statistical software package, Version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
POPULATION
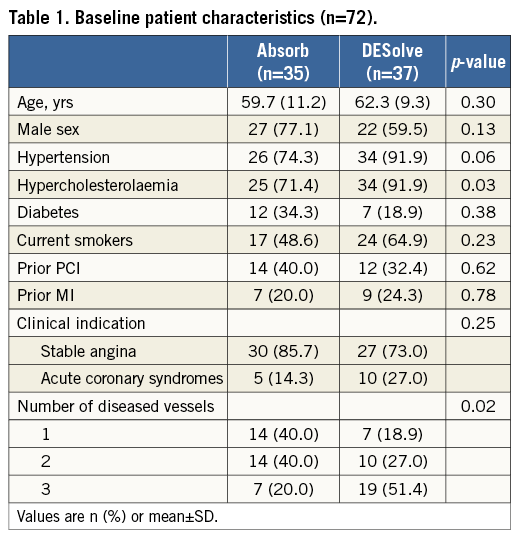
The population comprised 89 lesions in 72 patients, 35 patients in the Absorb group and 37 patients in the DESolve group. Baseline patient clinical characteristics are shown in Table 1.
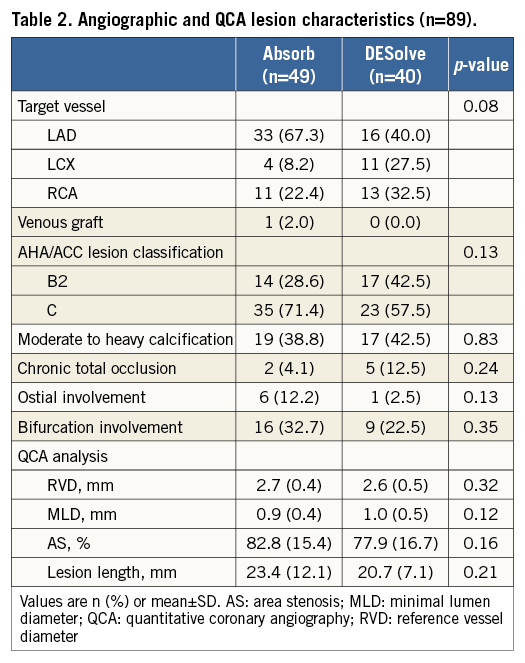
Angiographic and QCA baseline lesion characteristics are summarised in Table 2. The left anterior descending (LAD) was the target vessel in a large proportion of cases in both groups. RVD, MLD and lesion length, as assessed with QCA, were similar.
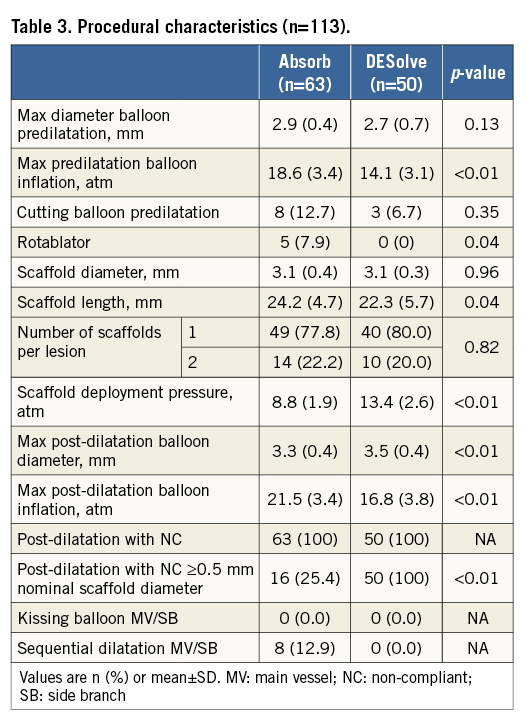
PROCEDURAL CHARACTERISTICS
Sixty-three Absorb and 50 DESolve were implanted. As shown in Table 3, significantly higher-pressure inflation for both pre- and post-dilatation was used with Absorb. On the other hand, DESolve were deployed at higher pressure (p<0.01).
OPTICAL COHERENCE TOMOGRAPHY FINDINGS
OCT findings are summarised in Table 4. A total of 1,914 cross-sections and 22,404 struts were analysed. Mean and minimal lumen area were similar in the two groups, while the maximal and minimal scaffold diameter were larger in DESolve (respectively, p=0.01 and p<0.01) without any significant difference in the mean scaffold area. The mean percentage of RAS was higher with Absorb (p<0.01).
There was no difference in the percentage of ISA between the two groups (p=0.96). The proportion of scaffolds with ISA at the distal end was higher in the DESolve group (p<0.01). DESolve were more eccentric as compared to Absorb (p<0.01).
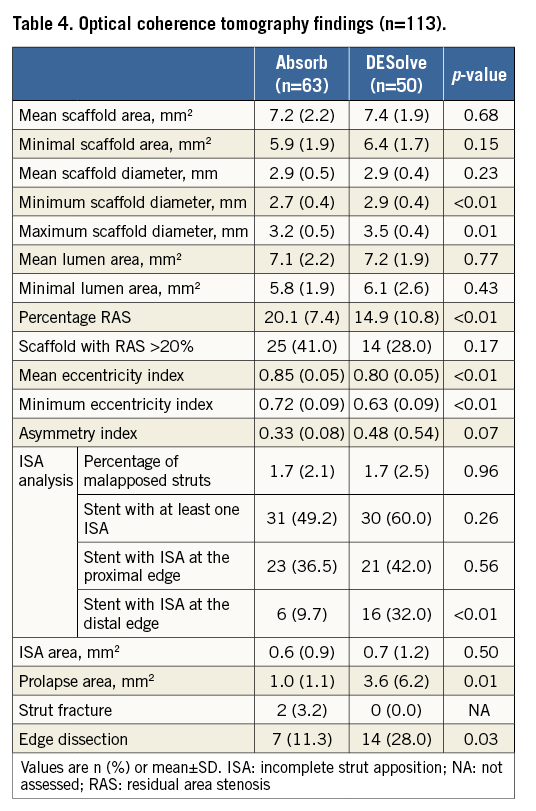
In the DESolve group, there was a larger prolapse area (p=0.01), but this did not have significant impact on the final lumen area, which was similar in both groups. OCT analysis showed 21 edge dissections (p=0.03), which were not apparent on the angiogram. Two patients in the Absorb group developed fractures at the site of scaffold re-crossing for side branch ostial dilatation.
CLINICAL FOLLOW-UP
Six-month clinical follow-up data were available for all patients. In the Absorb group, two patients underwent repeat target vessel revascularisation, one with repeat PCI and another with CABG.
In the DESolve group no events were observed.
Discussion
The principal finding stemming from our study is that both Absorb and DESolve BRS can be implanted with good acute results, according to imaging parameters generally accepted to define appropriate scaffold deployment11,12. These criteria are mainly derived from earlier studies, mainly using intravascular ultrasound (IVUS) in the era of bare metal stents (BMS) and first-generation drug-eluting stents (DES)15-17. In particular, a final minimal cross-section lumen area <5.5 mm2 or 6 mm2 and/or in-scaffold RAS >20% emerged as a risk for stent thrombosis16,17. These IVUS criteria were derived from trials enrolling simple A-B1 lesions, whereas our population comprised more complex B2-C lesions. Also, OCT has been shown to measure lower absolute areas than IVUS, both in vitro and in vivo18. Thus we believe that, whereas these criteria cannot be automatically applied to polymer-based scaffolds, the fact that the measured parameters in both the Absorb and DESolve groups were close or superior to the aforementioned cut-off is reassuring. In particular, the final MLA was very similar with Absorb and DESolve, suggesting that appropriate expansion can be achieved with both scaffolds.
Minimal and maximal scaffold diameter were slightly larger with DESolve and there was also a trend towards a larger minimal scaffold area and a numerically lower incidence of RAS >20% with DESolve. These results suggest a better expansion of the DESolve scaffold. Further efforts, with either in vitro experiments or in vivo studies, are advisable in order to confirm our observations. Indeed, we should be aware that these results might be partially biased by the use of larger balloons for post-dilatation with DESolve as compared to Absorb.
Interestingly, there was no strut fracture with DESolve. This was in accordance with a bench study by Ormiston et al in which the safe threshold for post-dilatation with the Absorb 3.0 mm was 3.8 mm at 20 atm while with the DESolve 3.0 mm it was 5.0 mm at 20 atm9.
Some stent properties influenced by material and design, such as conformability and flexibility, have previously been studied in various metallic stent platforms19-21. Our OCT study showed different post-procedural scaffold geometrical adaptation to the vessel wall with the two BRS. Mean and minimum eccentricity index were lower with DESolve despite the use of larger balloons for post-dilatation. These findings suggest that DESolve is more prone to asymmetric expansion. The clinical impact of asymmetric expansion is controversial and has been evaluated only with first-generation DES. An eccentricity value of 0.70 has been associated with favourable angiographic results at six-month follow-up in the MUSIC study22. Otake et al found that a low eccentricity index may be associated with thrombus formation after sirolimus-eluting stent implantation23. Nevertheless, Alfonso et al, analysing the IVUS data of 12 consecutive patients with stent thrombosis, found that the MUSIC criteria for asymmetric expansion were not fulfilled in any patient24.
As a general rule, BRS overexpansion should be performed with highly non-compliant balloons avoiding inflation of the delivery balloon at high pressure (>14 atm) which may result in non-uniform and asymmetric expansion along the scaffold length25.
The amount of tissue prolapse was significantly higher with DESolve than Absorb. This may be explained by the differences in scaffold design and possibly by the presence of larger scaffold cells with DESolve, especially when overexpansion is performed. Plaque prolapse probably explains the lack of any difference in the final MLA between the two scaffolds despite the presence of a trend towards a larger final minimal scaffold area with DESolve.
The clinical relevance of incomplete strut apposition is still debated and theoretically time-limited with BRS26-28. The thick struts of BRS have been shown to result in significant flow disturbance. Immediately after implantation, incompletely apposed struts act as obstacles, which disrupt the laminar flow and create an area of high shear rate which may promote scaffold thrombosis29. In our study, no difference in the incidence of incomplete strut apposition was observed between the Absorb and DESolve and no scaffold thrombosis occurred during six-month follow-up. Furthermore, the percentage of malapposed struts was considerably lower in comparison to data reported in previous OCT studies with first-generation DES30. The systematic use of post-dilatation and prompt correction with an appropriately sized balloon of any malapposition observed with OCT probably explains the low incidence of malapposition. In the DESolve group, the incidence of malapposition at the distal scaffold edge was slightly higher. Theoretically, minor malapposition with DESolve should be corrected whenever under the nominal diameter due to the self-expansion properties of this scaffold10. However, our study did not aim to assess the self-correction properties of the DESolve scaffold, since the final OCT pullback was performed immediately after the last scaffold post-dilation.
Study limitations
This was a small, non-randomised, retrospective study aimed at comparing the acute mechanical performance of two different PLLA scaffolds. The sample size does not allow any conclusion with regard to clinical endpoints. Minimal baseline and procedural differences might have affected the final results. The methods used for the assessment of stent eccentricity are not consistent in the literature; this aspect should be considered in the interpretation of our result regarding differences in scaffold geometry. The lack of a standardised OCT protocol for guidance of scaffold implantation together with the lack of universally accepted parameters for optimal scaffold implantation are a significant limitation of this study. OCT was used mainly in complex interventions, resulting in the selection of a large proportion of B2 and C lesions, which might be unusual for intervention with BRS.
Conclusions
Both Absorb and DESolve BRS can be implanted with good acute results. The final MLA was very similar with Absorb and DESolve, suggesting that appropriate expansion can be achieved with the two scaffolds. We observed minor differences in the expansion behaviour (in particular eccentricity), possibly due to the unique polymer properties of the two scaffolds.
| Impact on daily practice This first real-world comparison of two different BRS demonstrated that a satisfactory acute result could be obtained with both scaffolds. Meticulous implantation technique and OCT for guidance may have a significant impact on acute procedural results. At the beginning of a BRS implantation programme, intravascular imaging plays a pivotal role in the learning curve. Many different scaffolds will probably become available in the next few years, with intravascular imaging still shedding light on the mechanical properties of different platforms. However, it will be impossible to compare different BRS devices adequately with regard to their acute performance in the future unless well-defined, standardised criteria of imaging-based guidance can be applied. Hopefully, a deeper insight into the unique properties of different scaffolds will also translate into lesion-tailored selection of BRS. |
Conflict of interest statement
A. Mattesini has received speaker’s honoraria from Abbott Vascular. H. Nef has received speaker’s honoraria and an institutional grant from Abbott Vascular and Elixir Medical. C. Di Mario has received an institutional grant for the EXCEL study and the British ABSORB Registry from Abbott Vascular. The other authors have no conflicts of interest to declare.

