
Introduction
Bioresorbable scaffolds (BRS) were designed as an alternative to metallic stents with the goal of complete resorption to allow vascular remodelling and to decrease late clinical events associated with permanent implants1.
The DESolve® scaffold (Elixir Medical Corporation, Milpitas, CA, USA) is a next-generation scaffold with an early biodegradation and bioresorption profile that has demonstrated positive vascular remodelling at six months and favourable clinical outcomes2,3. The device is comprised of poly-L-lactic acid (PLLA) coated with a matrix of novolimus and a polylactide-based polymer. The strut thickness is 150 µm. We report the five-year clinical results with 18- and 36-month multimodality imaging data from the DESolve Nx trial.
Methods
The study design has been described previously3. Briefly, it was a non-randomised study enrolling patients with a de novo lesion with a maximum length of 14 mm in native coronary arteries between 2.5 and 3.5 mm in diameter. All patients were to undergo angiography at baseline and at six months with intravascular ultrasound (IVUS) and optical coherence tomography (OCT) imaging performed in a subset of patients at designated sites. Additional imaging was performed at 18 and at 36 months at two separate single centres. Linear mixed effect models were used to assess the temporal change of the quantitative coronary angiography (QCA), IVUS and OCT imaging parameters.
Results
The DESolve Nx Clinical Study enrolled a total of 126 patients. After the two-year follow-up, two patients were lost to follow-up. Two patients had a major adverse cardiac event (MACE) - a cardiac death at three years and a target vessel-related non-Q-wave myocardial infarction due to a proximal target segment stenosis treated with a stent at four years. The cumulative MACE rate at five years was 9.0% (Table 1). There were no reported definite scaffold thromboses.
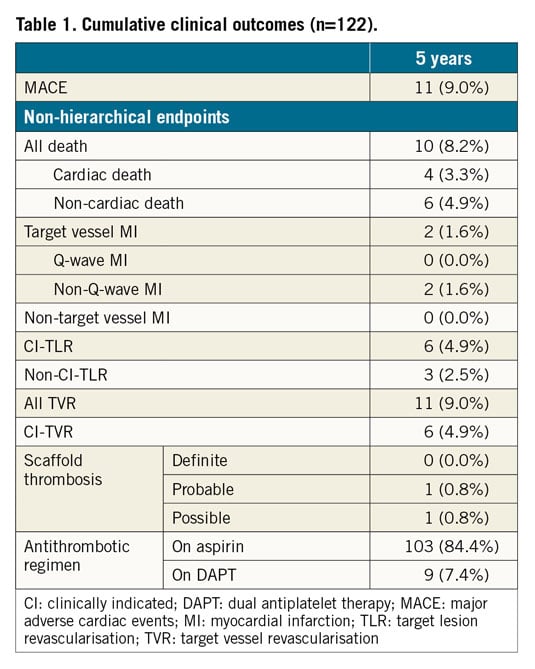
Imaging data are shown in Table 2. The scaffold was not visible by IVUS at 18 months or by OCT at 36 months (Figure 1). Neither IVUS nor OCT showed late-acquired incomplete strut apposition. Angiographically, the lumen loss did not change significantly (p=0.0568) over time; the in-scaffold minimum lumen diameter decreased significantly (p<0.0001). With IVUS, the mean lumen area did not change (p=0.3133); the minimum lumen area showed a decrease (p=0.0059). OCT showed a significant increase for the mean scaffold area (p<0.0001); both the mean and the minimum lumen area decreased significantly (p<0.0001).
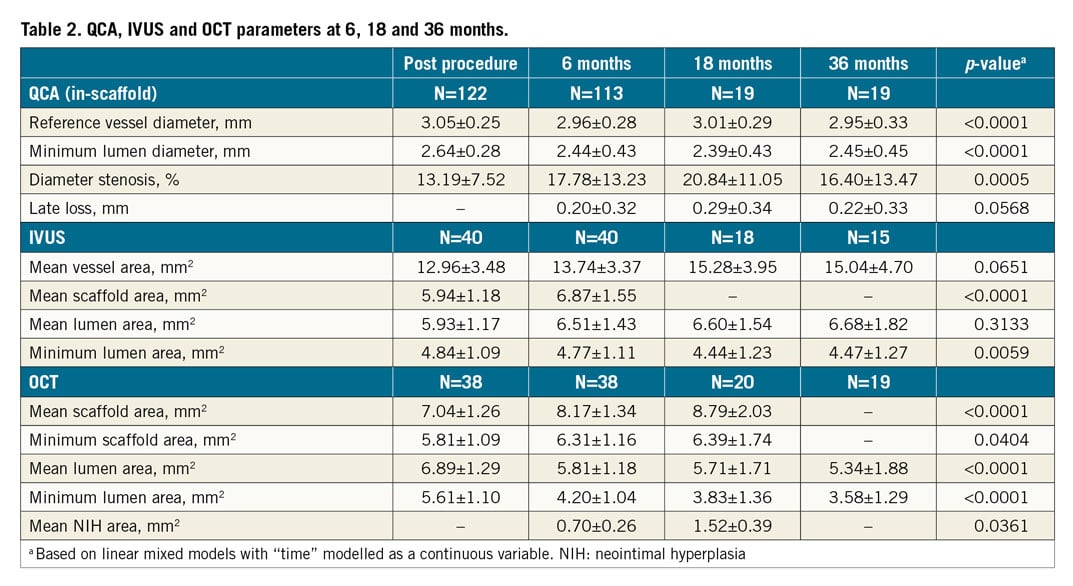
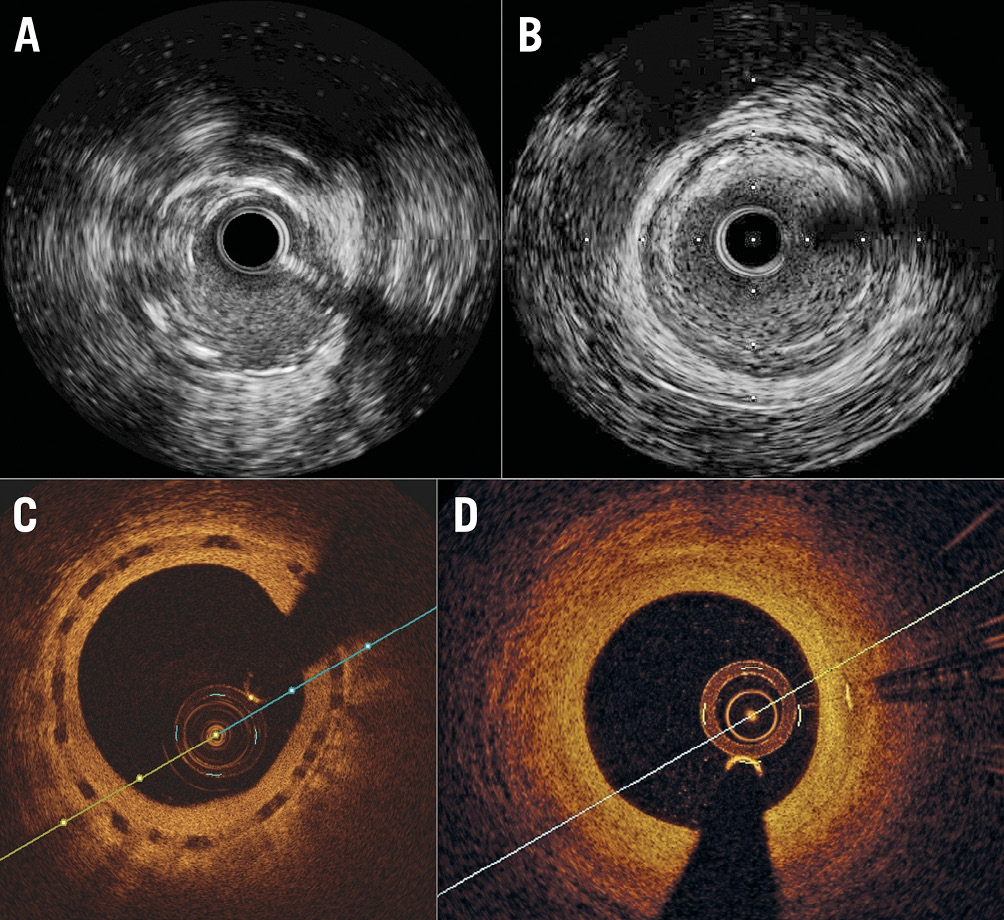
Figure 1. IVUS and OCT intracoronary imaging showing no visible struts at 18 and 36 months, respectively. A) IVUS at 6 months. B) IVUS at 18 months. C) OCT at 18 months. D) OCT at 36 months.
Discussion
The main findings of this study with the DESolve scaffold are 1) the good long-term clinical outcomes without a definite scaffold thrombosis, 2) the early resorption of the scaffold, and 3) enlargement of the scaffold.
The DESolve scaffold has been engineered with the design intention of full resorption within one to two years2. This study confirms the complete resorption of the scaffold by IVUS and OCT imaging at 18 months and three years, respectively. The mean scaffold area increased by both IVUS and OCT over time. This was first observed at six months, indicating an early start of the degradation process3. In contrast, for the Absorb™ scaffold (Abbott Vascular, Santa Clara, CA, USA), increase in mean scaffold area was observed after one year and struts were still visible by OCT at three years4. The optimal duration of the scaffold bioresorption process is speculative5, but the favourable clinical rates could be attributed to this early resorption. The numerical increase in mean lumen area by IVUS was not statistically significant, while it decreased by OCT. This discrepancy has been attributed to differences in resolution4.
Limitations
Important limitations of this study are the small sample size, absence of a control group and the non-complex patient population with primarily stable angina pectoris. The later imaging follow-ups were performed in a subset of patients at single sites only. The linear mixed effect model assumes a linear response which might not be precise.
Conclusion
In conclusion, the DESolve scaffold is completely resorbed at three years and has favourable clinical outcomes without definite scaffold thrombosis in selected non-complex patients with mostly stable angina pectoris.
|
Impact on daily practice This study confirmed the earlier resorption of the DESolve scaffold and showed a low event rate. Larger studies in complex patient populations that confirm these results are needed before starting to use the DESolve scaffold in daily practice. |
Funding
Elixir Medical (Milpitas, CA, USA).
Conflict of interest statement
S. Verheye has received speaker fees from Elixir Medical. The other authors have no conflicts of interest to declare.

