Practical concerns regarding poly-L-lactic acid (PLLA)-based bioresorbable scaffolds (BRS) are mostly related to the strut thickness of 150 µm. Implantation may cause more vessel injury as well as non-laminar flow and activation of platelet function. Furthermore, constraints of deliverability preclude BRS use in the presence of tortuosity or severe calcification. The PLLA-based novolimus-eluting DESolve® 100 scaffold (Elixir Medical Corporation, Sunnyvale, CA, USA) has a strut thickness of 100 µm. Complete degradation is achieved within two years. Furthermore, it has wider expansion ranges than other BRS and self-correction properties corresponding to nominal diameters, which can resolve minor malapposition.
In a mid-RCX stenosis, predilatation was performed with a non-compliant and a scoring balloon. Thereafter, a 2.5/28 mm BRS was implanted. Optical coherence tomography (OCT) revealed malapposition with a maximum vessel-wall distance of 0.33 mm and further edge dissection. Thus, a second 2.5/14 mm BRS was implanted distally. Another OCT run revealed a reduction of malapposition of the first BRS within 15 minutes: the vessel-wall distance decreased from 0.33 mm to 0.25 mm, and the minimal scaffold area increased from 3.94 mm2 to 4.28 mm2 (Figure 1). Nevertheless, malapposition was still present and post-dilatation was implemented with a non-compliant balloon. Final OCT confirmed a satisfactory result. The Online Appendix, Online Figure 1, Online Figure 2 and Moving image 1-Moving image 6 can be found online. The in vitro scaffold cross-sectional area under nominal pressure for the 2.5 mm DESolve 100 is 4.91 mm2, thus further self-correction is presumable. Self-correction appears reasonable especially in the presence of thrombi. Additional investigations will certainly be required for a conclusive proof-of-concept.
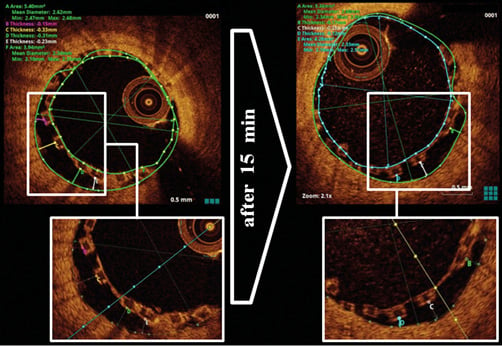
Figure 1. Evidence of malapposition and self-correction. Directly after implantation, malapposition was detected by OCT with a maximum vessel-wall distance of 0.33 mm. After 15 minutes, malapposition was decreased and the maximum vessel-wall distance was 0.25 mm.
Acknowledgements
We would like to acknowledge the assistance of Elizabeth Martinson, PhD, for helpful editing and advice in the preparation of the manuscript.
Conflict of interest statement
H. Nef has received speaker’s honoraria from Elixir Medical. The other authors have no conflicts of interest to declare.
Online data supplement
Online Appendix. Sequence of baseline and procedural imaging.
Online Figure 1. Scaffold implantation using the marker-over-marker technique.
Online Figure 2. Post-dilatation with a non-compliant balloon.
Moving image 1. Baseline angiography.
Moving image 2. Baseline OCT.
Moving image 3. Predilatation with a non-compliant balloon.
Moving image 4. Predilatation with a scoring balloon.
Moving image 5. Final angiography.
Moving image 6. Final OCT.
Online Appendix. Sequence of baseline and procedural imaging
Baseline angiography revealed a stenosis of the mid-RCX and another of the ostial RCX (Moving image 1). Additional optical coherence tomography imaging confirmed the angiographic findings (Moving image 2).
Predilatation was performed with a non-compliant balloon (2.5/20 mm) (Moving image 3) and with a scoring balloon (2.5/10 mm) at the site of lesion (Moving image 4). A 2.5/28 mm DESolve 100 scaffold was implanted. Due to distal edge dissection a further 2.5/14 mm DESolve 100 was implanted distally. The ClearStent technology demonstrated that implantation was performed in a marker-over-marker technique (Online Figure 1). Post-dilatation was performed with a non-compliant balloon (Online Figure 2) and final angiography showed TIMI 3 flow and a reasonable result (Moving image 5), which was further confirmed by OCT (Moving image 6).

Online Figure 1. Scaffold implantation using the marker-over-marker technique.
Online Figure 2. Post-dilatation with a non-compliant balloon.

