
Abstract
Aims: Our aim was to demonstrate the safety and efficacy of the Svelte sirolimus-eluting coronary stent-on-a-wire Integrated Delivery System (IDS) with bioresorbable drug coating compared to the Resolute Integrity zotarolimus-eluting stent with durable polymer in patients with de novo coronary artery lesions.
Methods and results: Direct stenting, particularly in conjunction with transradial intervention (TRI), has been associated with reduced bleeding complications, procedure time, radiation exposure and contrast administration compared to conventional stenting with wiring and predilatation. The low-profile Svelte IDS is designed to facilitate TRI and direct stenting, reducing the number of procedural steps, time and cost associated with coronary stenting. DIRECT II was a prospective, multicentre trial which enrolled 159 patients to establish non-inferiority of the Svelte IDS versus Resolute Integrity using a 2:1 randomisation. The primary endpoint was angiographic in-stent late lumen loss (LLL) at six months. Target vessel failure (TVF), as well as secondary clinical endpoints, will be assessed annually up to five years. At six months, in-stent LLL was 0.09±0.31 mm in the Svelte IDS group compared to 0.13±0.27 mm in the Resolute Integrity group (p<0.001 for non-inferiority). TVF at one year was similar across the Svelte IDS and Resolute Integrity groups (6.5% vs. 9.8%, respectively).
Conclusions: DIRECT II demonstrated the non-inferiority of the Svelte IDS to Resolute Integrity with respect to in-stent LLL at six months. Clinical outcomes at one year were comparable between the two groups. ClinicalTrials.gov Identifier: NCT01788150
Introduction
Drug-eluting stents (DES) are the standard of care for most percutaneous coronary interventions (PCI), particularly as the latest-generation DES incorporate more biocompatible polymers to mitigate stent thrombosis risk. However, simplifying stent delivery and reducing the catheter size necessary to deliver DES present an opportunity to reduce periprocedural complications while enhancing procedural efficiency. Downsizing catheters facilitates transradial intervention (TRI) in more patients, reducing the risk of bleeding complications, morbidity and mortality1, especially in ACS patients2,3, while improving time to ambulation, patient comfort and time and cost of PCI4,5. Direct stenting streamlines stent delivery and conveys important patient benefits, including reduced radiation exposure and intervention time6, myocardial infarction (MI) and death7, compared with conventional stenting in suitable lesions8. It also reduces the potential for mismatch between the section of the artery predilated and the section actually stented, a relevant consideration for long-term safety.
The Svelte SLENDER coronary stent-on-a-wire Integrated Delivery System (IDS™) (Svelte® Medical Systems, New Providence, NJ, USA) employs a thin (81 μm) L605 cobalt-chromium stent with an ultra-low crossing profile (as low as 0.029”), precludes use of conventional guidewires and predilatation balloons and can be used with small calibre catheters, including 4 Fr diagnostic catheters9. Elastic balloon control bands (BCBs) enveloping the low-compliant delivery balloon shoulders provide a smooth transition zone over the leading edge of the stent, minimising stent-to-vessel contact during delivery and deployment. The Svelte IDS has further demonstrated the potential to lower procedure times, contrast use, radiation exposure and total procedural costs, as suggested in prior studies utilising a bare metal stent version of the Svelte IDS10. Recently, a novel, fully bioresorbable amino acid drug carrier eluting sirolimus was added to this platform and was shown to be safe and effective in a first-in-human study (DIRECT I) enrolling 30 patients11.
This multicentre study was undertaken to assess prospectively the non-inferiority of the Svelte sirolimus-eluting coronary stent-on-a-wire IDS with bioresorbable drug coating compared to the Resolute Integrity™ zotarolimus-eluting stent (Medtronic, Minneapolis, MN, USA) with durable polymer in terms of six-month angiographic in-stent LLL.
Methods
STUDY DESIGN AND PATIENT SELECTION
Patients ≥18 years of age exhibiting clinical evidence of ischaemic heart disease, stable or unstable angina, silent ischaemia and/or a positive functional study and eligible for PCI with a target lesion stenosis ≥50% and <100% by visual estimate were randomised 2:1 to receive the Svelte IDS or Resolute Integrity. Up to two coronary lesions located in different major epicardial vessels could be treated, though only one lesion, designated as the target lesion, could be treated with the study device. Target lesions were ≤20 mm in length by visual estimate in vessels with reference vessel diameter (RVD) ≥2.5 mm and ≤3.5 mm. Non-target lesions had to be successfully treated with non-study stent(s) prior to treatment of the target lesion.
Clinical exclusion criteria included patients with myocardial infarction within 72 hours of index PCI, left ventricular ejection fraction ≤30%, serum creatinine >170 μmol/L or life expectancy less than one year. In patients with ST-segment elevation myocardial infarction (STEMI), the culprit lesion must have been successfully treated and the patient must have remained haemodynamically stable for a minimum of 72 hours prior to PCI. Patients with non-ST-segment elevation myocardial infarction (NSTEMI) could be included if troponin levels were normal within 24 hours prior to the procedure. Angiographic exclusion criteria included total occlusion (TIMI 0 or 1 flow), angiographic evidence of thrombus, excessive tortuosity, heavy calcification, target lesion supplied by a bypass graft, ostial target lesion (<5 mm from vessel origin or within left main artery), target lesions involving >2 mm side branches or unprotected left main coronary artery disease (>50% stenosis). All procedural details were prospectively collected via electronic case report forms (CRF) and monitored.
The protocol was approved by the local ethics committee, and written informed consent was obtained from all patients before inclusion in the study.
STUDY DEVICE
The Svelte IDS has three unique features: i) fixed-wire technology providing an ultra-low profile delivery system; ii) a new class of bioresorbable drug carrier; iii) a low-compliant delivery balloon with proprietary balloon control bands.
As described previously, the Svelte IDS integrates a guidewire with a stent delivery system to achieve an ultra-low crossing profile (as low as 0.029”) to downsize access-site size and facilitate transradial PCI and direct stenting. The system has a peel-away insertion tool to prevent any damage to the fixed-wire tip during introduction of the system.
The Svelte stent is coated with a thin (6 μm), fully bioresorbable drug carrier (DSM, Heerlen, The Netherlands) composed of naturally occurring amino acids eluting 213 μg/cm2 of the well-studied compound sirolimus (3.0×18 mm stent drug dose=130 μg). Extensive animal testing has demonstrated that sirolimus elution follows classic unidirectional (Fickian) diffusion kinetics, with approximately 80% of sirolimus released within 30 days and complete drug elution achieved in 60 days11. The drug carrier is resorbed in predictable and gradual fashion via enzymatic surface erosion (enzymolysis) over nine to 12 months, leaving only an endothelialised, bare metal stent behind. Resorption via enzymolysis limits significant pH change (and resultant activation of the complement cycle) and burst release, mitigating short- and long-term hyperplastic inflammatory response. The drug carrier demonstrates high mechanical integrity, a particularly relevant consideration with direct stenting.
The Svelte IDS delivery balloon includes proximal and BCBs to protect the leading edges of the stent from contact with the catheter or vessel wall during delivery. The BCBs are designed to control balloon expansion, limiting balloon shoulder growth at high atmospheres and “dog-boning”, thereby mitigating the risk of edge dissection and additional vessel trauma. The low-compliant balloon increases in diameter by approximately 0.25 mm from nominal to rated burst pressure (nominal pressure: 11 atm; rated burst pressure: 18 atm; mean burst pressure: 26 atm), allowing high-pressure dilatation(s) and often obviating the need for a separate non-compliant post-dilatation balloon.
PROCEDURE
Informed consent was obtained prior to any study-specific procedures. Patients began aspirin (75 mg) and clopidogrel (up to 600 mg) or prasugrel (60 mg) regimens within 24 hours prior to the index procedure. After insertion of the radial or femoral arterial sheath, heparin or bivalirudin was administered and supplemented as needed to maintain an ACT of ≥250 seconds (≥200 seconds if a glycoprotein IIb/IIIa inhibitor was used) throughout the interventional portion of the procedure. Following intracoronary injection of nitroglycerine (100-200 mcg), baseline angiography of the target vessel was performed to determine lesion eligibility.
Patients randomised to the Svelte IDS group were treated with a direct stenting strategy where feasible; crossover to a predilatation strategy was permitted, if deemed necessary by the investigator. Stenting strategy was at the investigator’s discretion for patients randomised to the Resolute Integrity group. Stent diameters available for both stents were 2.5, 3.0, and 3.5 mm. The Svelte IDS was available in 18 and 23 mm lengths and the Resolute Integrity was available in 18 and 22 mm lengths.
After discharge, clinical follow-up was scheduled at one, six and 12 months post PCI and annually thereafter for five years. Angiographic follow-up was scheduled for six months after index PCI. Post-discharge antiplatelet medications included clopidogrel (75 mg daily) or prasugrel (up to 10 mg daily) for a minimum of six months; aspirin (≥75 mg daily) was recommended indefinitely.
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA) ANALYSIS
Coronary angiography was conducted at six months. At follow-up, the same angiographic angles were used as at baseline. An independent core laboratory (Cardialysis, Rotterdam, The Netherlands) was used to assess all angiographic data and was masked to treatment allocation.
OPTICAL COHERENCE TOMOGRAPHY
Stent evaluation post procedure and at six-month follow-up in 30 patients treated with the Svelte IDS was conducted at predetermined sites with OCT capability. OCT with pullback from 10 mm distal to the stent to 10 mm proximal to the stent was performed.
STUDY ENDPOINTS, DEFINITIONS AND SAMPLE SIZE
The primary endpoint was angiographic in-stent late lumen loss (LLL, defined as the difference between the post-index procedure minimal lumen diameter [MLD] and the follow-up MLD) at six months post procedure. Secondary angiographic endpoints included in-stent and in-segment angiographic binary restenosis, in-stent and in-segment MLD and in-segment LLL at six months post procedure. In-segment was defined as measurements either within the stented segment or 5 mm proximal and distal to the stent edges. Expected balloon diameter was calculated as the theoretical diameter at the maximal pressure from the compliance chart provided by the balloon manufacturer.
OCT findings included neointimal hyperplasia (% lumen volume), strut coverage (% of struts malapposed, protruding non-covered, protruding covered, non-protruding covered) at six months post procedure.
The secondary clinical endpoint (unpowered) was target vessel failure (TVF, defined as the composite of cardiac death, target vessel MI [Q or non-Q-wave], or clinically driven target vessel revascularisation [TVR]). Other secondary clinical endpoints included death, MI, device-oriented major adverse cardiac events (MACE, defined as the composite of cardiac death, all MI and all TLR), target lesion revascularisation (TLR, defined as repeat PCI or coronary bypass grafting of the target lesion), target lesion failure (TLF, defined as cardiac death, target vessel MI and clinically driven TLR) and stent thrombosis at 30 days, six months and annually at years one to five post PCI. Clinically driven TLR and TVR were defined according to the Academic Research Consortium (ARC) standards12. Periprocedural and spontaneous myocardial infarction were defined according to the third universal definition13.
Device success was defined as attainment of <30% final residual stenosis of the target lesion using the assigned device, and direct stenting success was defined as attainment of <30% final residual stenosis of the target lesion without predilatation. Lesion success was defined as attainment of <30% final residual stenosis of the target lesion using any stent, with or without other interventional devices, and procedure success was defined as lesion success with no in-hospital MACE. Inappropriate device use was defined as implantation of the device without following the device instructions for use (e.g., introducing the Svelte IDS without using the protective peel-away insertion tool).
STATISTICS
Sample size was calculated based on a non-inferiority design, with the study intending to show non-inferiority of the Svelte IDS compared to the Resolute Integrity in the primary efficacy endpoint of in-stent LLL. Statistical analysis was performed using a one-sided t-test (α=0.025) comparing the mean in-stent LLL in both groups with a non-inferiority margin of 0.18. Standard deviations of both groups were assumed to be equal at 0.32. To demonstrate non-inferiority of mean in-stent LLL at six months in both groups with a power of 80%, a total of 138 subjects had to be included in the study. To account for angiographic follow-up loss of 15%, a total of 159 subjects were enrolled in the study. Continuous variables were presented as mean and standard deviation while categorical variables were presented as number and percentage. To compare the baseline angiographic and clinical characteristics between the two groups, chi-square or Fisher’s exact tests were used for categorical variables, while the Student’s t-test was used for continuous variables. For the t-test, variances were pooled if the test on the equality of variances had a p-value >0.05. All statistical analyses were performed using SAS (version 6.12 or higher) (SAS Institute Inc., Cary, NC, USA). The primary analysis sample was based on the principle of intention-to-treat.
Results
PATIENT, LESION AND PROCEDURAL CHARACTERISTICS
A total of 159 patients were treated at 18 sites in Europe. Baseline patient and lesion characteristics were similar between the two groups (Svelte IDS, n=108; Resolute Integrity, n=51), except for smoking status and ACC/AHA lesion type, as reported in Table 1. There was also a trend towards inclusion of a greater number of lesions with moderate to heavy calcification in the Svelte IDS group (21.7%) than in the Resolute Integrity group (9.8%). Approximately 20% of patients had diabetes. Two thirds of interventions were performed via the transradial approach and direct stenting was attempted in 92.6% and 88.2% of procedures in the Svelte IDS group and Resolute Integrity group, respectively. Five patients crossed over from the Svelte IDS to the control group, while no Resolute-treated patients crossed over: three were due to failure to follow the Svelte IDS instructions for use leading to product removal and crossover to control device, and two were due to inability of the study device to cross the target lesion. Post-dilatation was performed in approximately one quarter of patients in both arms (Table 1). There were no difficulties reported in re-crossing deployed Svelte stents. There were no clinical sequelae in these patients.
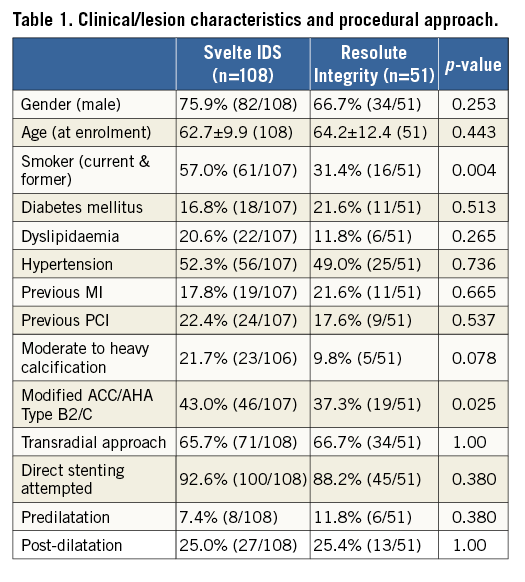
PROCEDURAL OUTCOMES
Device failure rates (>30% DS post procedure) were 1.9% and 0% in the Svelte IDS and Resolute Integrity groups, respectively; 37% of operators were first-time users of the Svelte IDS. There were no significant differences in lesion success or procedural success between the Svelte IDS and Resolute Integrity groups (96.3% and 100%; p=0.31, and 94.4% and 94.1%; p=1.00, respectively). In the population excluding patients with inappropriate device use (n=5) or optional OCT investigation (n=30), procedural time (i.e., the time from guiding catheter insertion to withdrawal) was similar between the Svelte IDS and Resolute Integrity groups (30±18.5 minutes vs. 29±19.6 minutes; p=0.431, respectively). Device time (i.e., the time from guidewire insertion to withdrawal) in the Svelte IDS group was significantly less compared to the Resolute Integrity group (8±7.6 minutes vs. 11±10.3 minutes; p=0.027, respectively).
QUANTITATIVE CORONARY ANGIOGRAPHY
Pre-procedure, post-procedure, and six-month angiographic outcomes are reported in Table 2. There were no significant differences in pre-procedural angiographic findings. Baseline mean RVD was 2.68 mm and 2.74 mm in the Svelte IDS and Resolute Integrity groups while lesion length was 13.4 mm and 13.7 mm, respectively. The nominal size of stents and post-dilatation balloons was not different between the two groups. The expected balloon diameter of the last balloon used (i.e., the device balloon or post-dilatation balloon) was, however, larger in the Resolute Integrity group than in the Svelte IDS group.
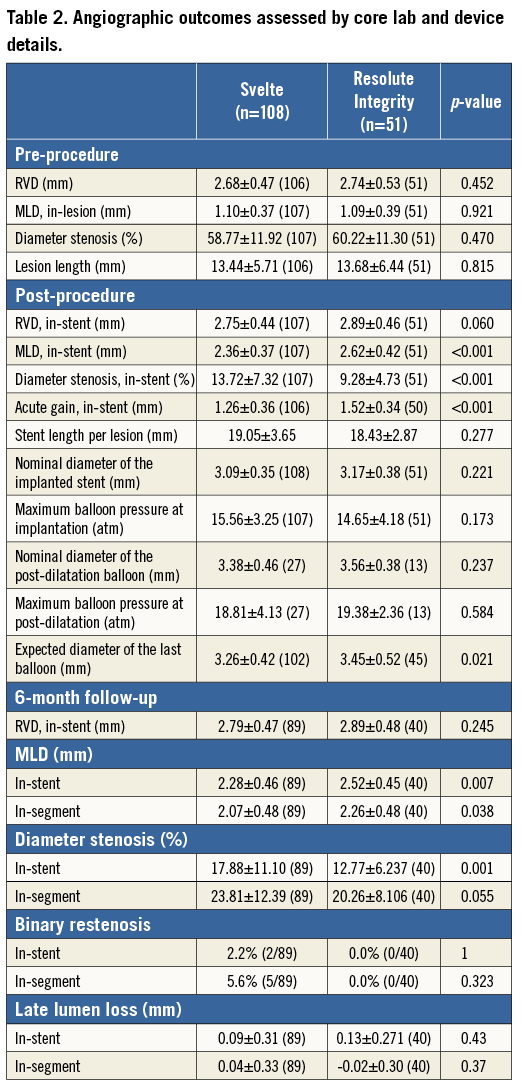
There were significant differences in post-procedural angiographic findings, including smaller acute gain, post-procedural MLD and % diameter stenosis in the Svelte IDS group compared with the Resolute Integrity group, as seen in Figure 1A. Dissections were noted via angiography in four patients (Svelte IDS=four [3.7%], Resolute Integrity=zero [0.0%], p=0.306): one Type A at the lesion site, one Type A distal to the stent, one Type A proximal to the stent and one Type B proximal to the stent. All dissections resulted in the placement of an additional non-study stent in the affected area without further sequelae.
At six-month follow-up, significant differences in MLD and % DS remained; however, there were no significant differences observed with in-stent LLL or binary restenosis, nor were there any significant differences observed in clinical outcomes at one year. The primary endpoint of six-month in-stent LLL, described in Figure 1B, demonstrates non-inferiority of the Svelte IDS to Resolute Integrity (p=0.43; difference and 95% confidence interval –0.05 [–0.16, 0.07] mm; p for non-inferiority <0.0001).
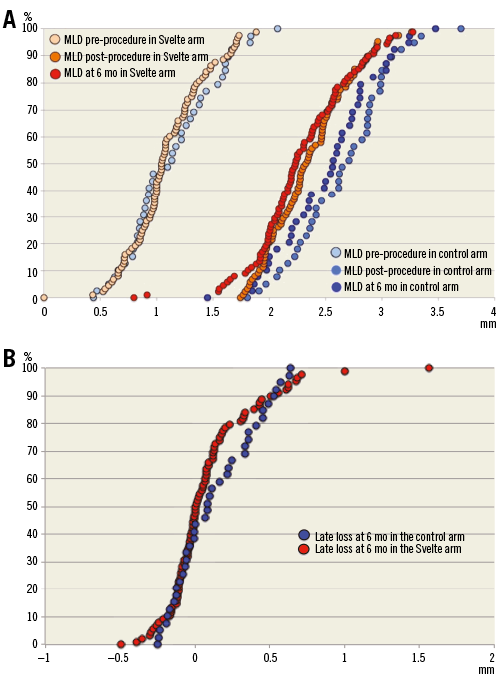
Figure 1. Angiographic outcome cumulative frequency curves. A) Cumulative frequency curves of angiographic MLD pre-procedure, post-procedure and at six months in the Svelte IDS and control groups, in patients with six-month angiographic follow-up. B) Cumulative frequency curves of angiographic late loss at six months in the Svelte IDS and control groups, in patients with six-month angiographic follow-up.
OPTICAL COHERENCE TOMOGRAPHY
Six-month OCT results were available in 22 Svelte IDS patients. Neointimal hyperplasia area was 0.89±0.33 mm2 and neointimal hyperplasia volume obstruction was 11.3%. Malapposed struts were detected in 0.7±1.9% of struts. Strut coverage on average was 94.2±9.0%.
Clinical outcomes up to one-year follow-up
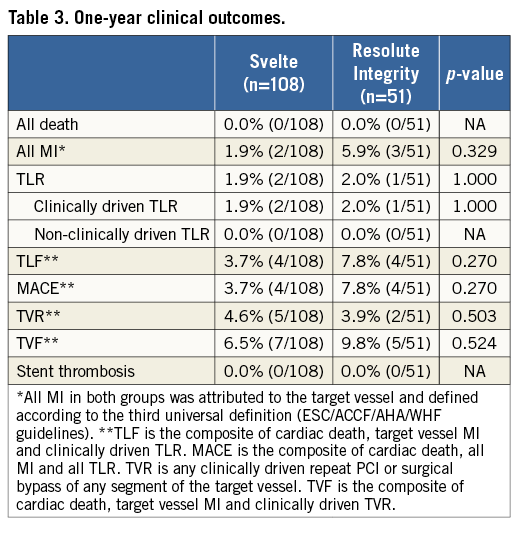
At one year, vital status was known for 97.5% of patients, with complete clinical follow-up available in 91.2% of patients. TVF occurred in seven patients (6.5%) in the Svelte IDS group and in five patients (9.8%) in the Resolute Integrity group (p=0.524) at one year. No significant differences were observed in TLR or TVF rates between the treatment groups (Figure 2). There were also no differences in the components of TVF between the Svelte IDS and Resolute Integrity groups up to one year: cardiac death (0.0% vs. 0.0%; p=NA); target vessel MI (1.9% vs. 5.9%; p=0.329); clinically driven TVR (2.8% vs. 2.0%; p=1.00), respectively. Table 3 shows one-year clinical outcomes by intention-to-treat.
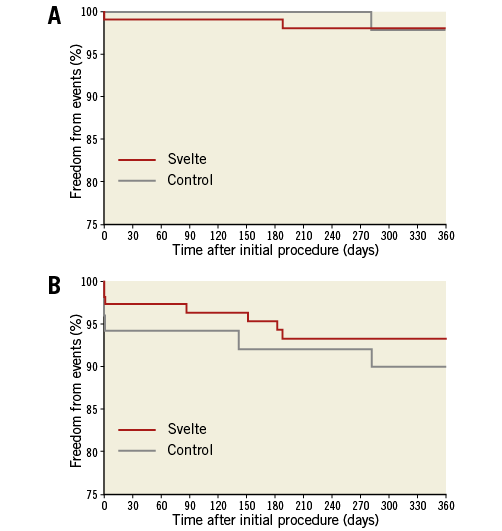
Figure 2. Freedom from clinical events up to one year. A) Kaplan-Meier curves demonstrating freedom from TLR in the Svelte IDS and control groups up to one year. B) Kaplan-Meier curves demonstrating freedom from TVF (cardiac death, target vessel myocardial infarction [Q or non-Q-wave], or clinically driven TVR) in the Svelte IDS and control groups up to one year.
There were no reports of stent thrombosis in either group up to one year. At one year, 71% of patients received dual antiplatelet therapy with either clopidogrel (64%) or prasugrel (7.5%). One patient was not on any antiplatelet therapy, one patient was on antiplatelet therapy without aspirin, one patient was on an anticoagulant only, five patients were taking aspirin only and 14 patients were taking an anticoagulant in conjunction with the ASA guidelines for atrial fibrillation.
Discussion
This is the first randomised trial demonstrating that the Svelte IDS, a novel, coronary stent-on-a-wire with bioresorbable drug coating technology, is non-inferior to the Resolute Integrity DES with respect to inhibition of neointimal proliferation. With a mean in-stent LLL of 0.09±0.31 mm at six months, the Svelte IDS appears to achieve outcomes similar to other “limus” DES. While not powered to detect differences in clinical outcomes, one-year TVF and TLF were numerically lower with the Svelte IDS compared to the Resolute Integrity DES.
The lack of any stent thrombosis in the first 137 patients treated with Svelte IDS across the DIRECT I and DIRECT II studies is a reassuring initial signal related to safety. OCT findings from this study indicated that more than 99% of stent struts were apposed to the vessel wall at six-month follow-up, also a positive indicator for long-term safety.
As the Svelte IDS is low-profile and specifically designed for direct stenting through “slender” catheters, identifying a drug coating capable of maintaining mechanical integrity and drug release kinetics even in challenging anatomy or prolonged interventions is critical. Novel elastic BCBs located on the proximal and distal shoulders of the balloon and slightly greater in profile than the stent itself also help to protect the drug coating during delivery, offering a smooth leading edge while limiting longitudinal growth and balloon-vessel contact during stent deployment.
With a rated burst pressure of 18 atm and approximately 0.25 mm of increase in the balloon diameter from nominal to rated burst pressure, the Svelte delivery balloon is less compliant than conventional stent delivery systems. The benefits of utilising this balloon, particularly at higher pressures, include greater control during stent expansion and reduced need for post-dilatation balloons, saving time and resources in certain cases. In the present study, however, the average pressure used during stent deployment was similar across both groups. About 25% (27 of 108) of Svelte IDS-treated patients underwent post-dilatation, with 17 of 27 patients using a non-study balloon. About 25% (13 of 51) of the Resolute Integrity-treated patients underwent post-dilatation with a non-study balloon.
When the theoretical expected stent diameter was calculated using the pressure and compliance chart provided by the manufacturer, the expected diameter of the last balloon used (either device delivery balloon or post-dilatation) was smaller in the Svelte IDS arm than in the Resolute Integrity arm (3.26±0.42 mm vs. 3.45±0.52 mm, p=0.021), suggesting that operators in the Svelte IDS arm did not expand the stent as much as in the Resolute Integrity arm. This could explain the significantly smaller acute gain (1.26±0.36 vs. 1.52±0.34, p<0.001) and smaller in-stent MLD (2.36±0.37 vs. 2.62±0.42, p<0.001) achieved in the Svelte IDS group compared with the Resolute Integrity group. Application of higher pressures at the time of device implantation and appropriate post-dilatation potentially improve the acute performance of the study device.
Retrospective analysis confirmed that these outcomes were not influenced by lesion calcification, disproportionate use of post-dilatation balloons or stent recoil. Rather, this probably reflects inexperience with Svelte delivery balloon compliance on the part of the investigators, one third of whom were first-time users of the Svelte IDS, reiterating the need for proper training to optimise use of the Svelte balloon technology. Importantly, no flow-limiting dissections occurred with the Svelte IDS, which is consistent with prior studies and should be reassuring to first-time users.
In DIRECT II, the transradial approach was used in two thirds of cases with a high rate of procedural success. TRI, which is already prevalent in Europe, is steadily growing within the United States, given its association with reduced access-site complications, shorter procedure and patient ambulation times and enhanced patient comfort6,14-18. However, reducing device profiles remains a priority given the increased incidence of procedural complications, including radial artery occlusion, with larger catheter sizes. The low system profile of the Svelte IDS enables “slender” PCI and adequate contrast flow with vessel visualisation during stent implantation, even in smaller calibre catheters. Further, the benefits of direct stenting in appropriate lesions (reduced mortality, exposure to radiation, contrast administration and use of ancillary products) are well documented10.
In cases excluding OCT and device misuse in DIRECT II, procedural times were similar between the Svelte IDS (n=71) and Resolute Integrity (n=51) groups (30±18.5 minutes vs. 29±19.6 minutes; p=0.431, respectively); however, the Svelte IDS demonstrated significantly shorter mean device time compared with the Resolute Integrity (8±7.6 minutes vs. 11±10.3 minutes; p=0.027, respectively). A sub-analysis of the top five enrolling sites indicated that, after physicians gain experience of at least five cases with the Svelte IDS, both procedure (22±12.3 minutes vs. 30±19.6 minutes; p=0.039, respectively) and device (4±3.3 minutes vs. 11±10.3 minutes; p=0.008, respectively) times are reduced. A total of 25 and 55 guidewires and two and 22 non-study balloon catheters were used in the Svelte IDS and Resolute Integrity groups, respectively. Particularly when considering the 2:1 randomisation scheme of the study, the potential for meaningful reductions in material costs becomes apparent and is consistent with previous reports10. These time and cost savings may prove increasingly relevant in the current healthcare environment.
Study limitations
This study was not powered to detect differences in clinical outcomes. In addition, operators were not blinded to treatment assignment, which could have influenced the decision to reintervene during follow-up. However, the use of an independent CEC to adjudicate reinterventions as clinically driven was used to minimise potential operator bias. Further, six-month angiographic follow-up may not represent peak neointimal hyperplasia response following DES implantation which has been seen with some DES between six and 24 months. While animal model data suggest that the Svelte DES is not associated with delayed neointimal proliferation, longer-term follow-up is needed to confirm this in clinical trials. Finally, subset analyses with experienced users indicate that the Svelte IDS was associated with reduced procedure and device time as well as adjunctive product use. Though these parameters were prospectively collected, this was not a pre-specified analysis and must therefore be confirmed in a dedicated study.
Conclusions
The DIRECT II study demonstrated non-inferiority of the Svelte IDS to the Resolute Integrity DES with respect to in-stent LLL at six months. Clinical outcomes, including TVF and TLF, were comparable at six- and 12-month follow-up. The combination of a fixed-wire delivery platform, bioresorbable drug carrier and specialised balloon technology suggests that the novel Svelte IDS is a safe and effective treatment for coronary artery disease.
| Impact on daily practice The DIRECT II trial demonstrates the safety and efficacy of the Svelte IDS. As a fixed-wire DES system, the Svelte IDS attains ultra-low profiles and requires less catheter back-up support than conventional rapid-exchange systems, allowing the downsizing of catheters. Facilitating “slender” intervention and direct stenting streamlines the steps necessary to complete PCI, improving patient comfort while reducing procedural time and cost. |
Guest Editor
This paper was guest edited by Carlo di Mario, MD, PhD, FESC, FACC, FRCP, FSCAI; NIHR Biomedical Research Unit, Royal Brompton & Harefield NHS Foundation Trust, London, UK.
Funding
This trial is sponsored by Svelte Medical Systems, Inc.
Conflict of interest statement
S. Verheye, S. Khattab and P. Stella report institutional grants from several device companies, including Svelte Medical Technologies. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

