- meta-analysis
- biodegradable-polymer drug-eluting stents
- permanent-polymer drug-eluting stents
- target lesion revascularisation
- definite stent thrombosis
Abstract
Aims: Different biodegradable-polymer drug-eluting stents have not yet been systematically analysed. We sought to; 1) evaluate the risk of target lesion revascularisation (TLR) and definite stent thrombosis (DST) among different groups of biodegradable-polymer (BioPol) DES, and 2) to compare them with permanent polymer (PermPol) DES.
Methods and results: We searched PubMed and relevant sources from January 2005 until October 2010. Inclusion criteria were (a) Implantation of a drug-eluting stent with biodegradable polymer; (b) available follow-up data for at least one of the clinical end-points (TLR/DST) at short term (30 days) and/or mid-term (one year). A total of 22 studies, including randomised and observational studies, with 8264 patients met the selection criteria; nine studies (2042 patients) in whom biodegradable-polymer sirolimus eluting stents (BioPol-SES) were implanted, eight studies (1731 patients) in whom biodegradable-polymer paclitaxel eluting stents (BioPol-PES) were implanted, and seven studies (4491 patients) in whom biodegradable-polymer biolimus-A9 eluting stents (BioPol-BES) were implanted. At 30 days, there was a higher risk of DST (p=0.04) and subsequently TLR (p=0.006) in the BioPol-BES compared to BioPol-SES, with no significant difference in the other stent comparisons. At 1-year, there was higher risk of TLR in the BioPol-PES (p= 0.01), and the BioPol-SES (p=0.04) compared to BioPol-BES. One-year stent thrombosis was not statistically different between the studied groups (overall p= 0.2). In another analysis comprising seven randomised trials comparing BioPol-DES (3778 patients) and PermPol-DES (3291 patients), the risks of TLR and stent thrombosis at 1-year were not significantly different (p= 0.5 for both).
Conclusions: Performance of different BioPol-DES seems to vary from each other. The short- and mid-term success rates may not be superimposable. Furthermore, they may not be necessarily better than PermPol-DES.
Introduction
Drug-eluting stents (DES) represent a major breakthrough in the field of percutaneous coronary interventions (PCI), since they have dramatically reduced the need for repeated revascularisation procedures1,2.
Along with the increasing number of patients receiving DES and the availability of long-term follow-up data, concern has arisen regarding the safety of these devices with the potential for increased inflammatory response and stent thrombosis which could have life-threatening consequences3-5.
Polymers used for the delivery of antirestenotic agents have been accused in the development of late stent thrombosis. This is thought to be secondary to polymer-induced inflammatory reaction, with delayed healing and re-endothelialisation of the DES6. Given these issues, more focus has been placed upon developing biodegradable polymers, which degrade over time, and therefore possibly eliminate the problems of polymer-induced inflammation.
In some cases, findings from preclinical studies can be misinterpreted, especially in cases where the drug may be toxic. The polymer may be blamed for the inflammation or excessive fibrin deposition and lack of endothelialisation. Yet the difference in pharmacokinetics and antirestenotic efficacy of the different drugs could also be held responsible for variation in clinical outcomes. Thus, a “polymer only” control is essential in distinguishing the culpability between polymer versus drug7. However, this is a control difficult to implement in clinical studies.
Various studies were conducted to test the clinical performance of a variety of biodegradable polymer-based stents eluting sirolimus (BioPol-SES), biolimus A9 (BioPol-BES) or paclitaxel (BioPol-PES).
The aims of the present meta-analysis were: 1) to compare the short-term (1-month) and mid-term (1-year) performance of sirolimus, biolimus A9 and paclitaxel biodegradable-polymer DES; and 2) to compare, where information was available, the 1-year performance of biodegradable polymer DES (BioPol-DES) with permanent polymer DES (PermPol-DES).
Methods
Eligibility and search strategies
To be included in this meta-analysis, studies had to meet the following criteria: a) implantation of a drug-eluting stent with biodegradable polymer; b) available follow-up data for at least one of the clinical endpoints at short-term (30 days) and/or mid-term (up to one year). Studies dedicated to specific lesion subsets including; left main stenting, bifurcation lesions, chronic total occlusions, long lesions, in-stent restenosis and venous grafts were excluded.
We searched PubMed, Web of Science, and Embase (OVID) from January 2005 and onwards for studies on biodegradable DES. The PubMed search strategy was formulated as the AND-combination of 1) DES terms and 2) terms denoting biodegradable or permanent polymer as follows: 1) “Drug-Eluting Stents”[Mesh] OR DES[tiab] OR ”drug-eluting stent”[tiab] OR ”drug-eluting stents”[tiab] OR ”drug eluted stent”[tiab] OR ”drug eluted stents”[tiab] OR “drug coated stent”[tiab] OR “drug coated stents”[tiab]; 2) “biodegradable polymer”[tiab] OR “permanent polymer”[tiab] OR “nonbiodegradable polymer”[tiab] OR biodegradable[tiab] OR bioabsorbable[tiab] OR “durable polymer” OR (“Polymers”[Mesh] AND (biodegradable[tiab] OR bioabsorbable [tiab] OR permanent[tiab] OR nonbiodegradable[tiab])). This search strategy was translated to the corresponding vocabulary of Embase and Web of Science. Relevant websites (http://www.tctmd.com, www.europcr.com) were searched for pertinent abstracts and expert slide presentations. The search was kept updated until October 2010. No language restriction was applied.
Data abstraction
Two investigators (T.A.N.A. and S.C.B.) independently extracted all data, and disagreements were solved in consultation with a third investigator (J.W.M.P.). From PubMed,144 papers were identified, 52 papers from Web of Science and 125 papers from EMBASE, and four additional clinical trials from relevant websites (total of 325 citations) (Figure 1). After reading the titles and abstracts, a total of 25 potentially relevant studies were initially identified from which 22 were eligible for inclusion.
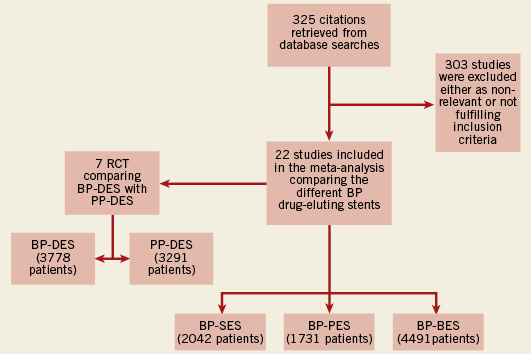
Figure 1. Flow diagram of the review process. Process of identification and selection of the studies for inclusion in the meta-analysis. BP:biodegradable polymer; PP: permanent polymer; SES: sirolimus-eluting stents; PES: paclitaxel-eluting stents; BES: biolimus eluting-stents; DES: drug-eluting stents
Definitions and endpoints
The clinical endpoints of the study were the rates of target lesion revascularisation (TLR) and definite stent thrombosis (DST) at 30days and one year follow-up. Even in the few studies with follow-up more than one year only the data at one year were used.
TLR was defined as percutaneous or surgical revascularisation of the target lesion. Definite stent thrombosis was defined, whenever available, according to the definition of the Academic Research Consortium8.
Statistical analysis
For the comparison of the three biodegradable-polymer stent types (BioPol-SES, BioPol-PES and BioPol-BES), 20 studies provided one patient series and two studies provided two patient series. Since there were only two studies with two series, we analysed the 24patient series as independent studies. For each patient series we extracted from the publications the number of events (TLR or DST within 30 days or one year) and the corresponding number of patients. For each stent type, type of event and follow-up period the exact 95% confidence interval for a binomial proportion was calculated and depicted in a forest plot. If the observed proportion was zero, the one-sided 97.5% confidence interval was given. To compare the incidences of the different types of events between the different stent types, we used random effects meta-analysis. More specifically, we used random intercept logistic regression with two dummy variables representing the three stent types, as described in Stijnen et al9. This analysis assumes that the between studies variance was equal for the different stent types. In the analysis, the random effects take into account the possibility that there may be many differences between the patient populations of the different studies, influencing the risks of the considered endpoints. To adjust for multiple comparisons, we first tested at α=0.05 the overall null hypothesis that all three stent types had equal incidence. If this test was significant, the three pair-wise tests were done at α=0.05. For the incidence of TLR within 30 days, the estimate of the between studies variance was zero. In that case the analysis reduces to ordinary logistic regression and we used exact tests and confidence intervals for the odds ratios.
Seven studies comprised trials comparing BioPol-DES with PermPol-DES. To make forest plots, we calculated exact 95% confidence intervals for the odds ratio except for studies in which less than two events in total were observed. We used random effects meta-analysis to estimate and test the overall odds ratio across studies. Due to the scarcity of data at 30 days among the included studies, we decided to assess the events at one year only. Because of the small numbers of events in some of the studies, the hypergeometric-normal model as described in Stijnen et al was used9.
All statistical analyses were performed using SAS statistical package version 9.1.3. The procedure NLMIXED was used for the random-effect meta-analysis.
Study quality assessment
This meta-analysis was especially designed to extract data from various types of available studies: observational studies presenting data about BioPol-DES; randomised clinical trials (RCTs) in which different BioPol-DES are compared among each other, and RCTs in which BioPol-DES is compared with PermPol-DES. Only for the latter category, was it of interest to perform an RCT study quality assessment. We have used the “Delphi list” for the quality assessment of RCTs as described by Verhagen et al10. In short, the Delphi list allocates “yes”, “no”, or “do not know” to a total number of nine questions. Quality of RCTs is defined as the likelihood of the trial design to generate unbiased results. When five or more questions are answered “yes”, the RCT is considered to have a low risk of bias. In a respective manner, RCTs may have unclear or high risk to cause bias.
Results
Trials and study characteristics
A total of 22 studies11-33 with a total of 8,264 patients were included in this meta-analysis (Table 1); nine trials11,13,14,17,21,23-25,30,32 (2,042 patients) with biodegradable polymer sirolimus eluting stents (BioPol-SES), eight trials12,20,22,23,27,28,31,32 (1,731 patients) with biodegradable polymer paclitaxel eluting stents (BioPol-PES) of which two were randomised trials against BioPol-SES23,32; and seven trials15,16,18,19,26,29,33 (4,491 patients) with biodegradable polymer biolimus A9 eluting stents (BioPol-BES). Many of the retrieved trials were observational non-randomised trials. The mean age of the participants in individual trials ranged from 53 to 67 years, with males representing the majority. The percentage of diabetics was 28% among the BioPol-SES, 26% among the BioPol-PES and 28% among the BioPol-BES. The recommended duration of thienopyridine therapy after stent implantation was variable between the studies; three months in two studies19,30, six months in 12 studies12-16,21,22,25-28,31,32, 12 months in five studies11,20,23,24,33, indefinitely in one study17 and unidentified in two studies18,29. The follow–up (FU) duration ranged from six months to 30 months, with only two studies with FU of six months11,24.
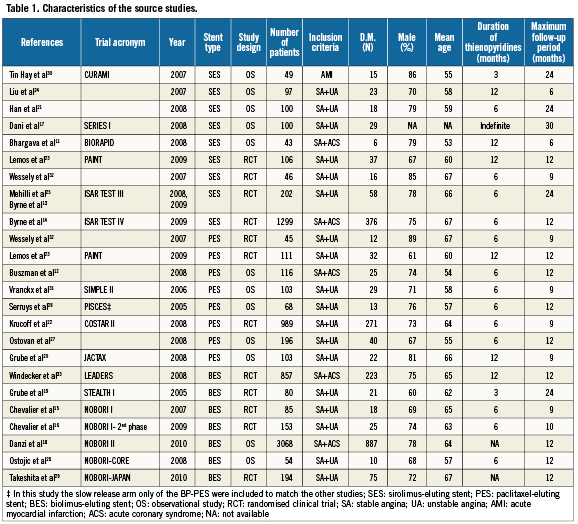
Among the included studies there were 10 randomised clinical trials (RCT)13-16,19,22,23,25,29,32,33; two studies were randomising BioPol-SES versus BioPol-PES23,32; there were two other studies with one randomising BioPol-SES versus permanent polymer sirolimus-eluting stent (PermPol-SES)13,25, and the other randomising BP-SES versus permanent polymer sirolimus (PermPol-SES) plus everolimus-eluting stents (PermPol-EES)14; one study randomising BioPol-PES versus permanent polymer paclitaxel-eluting stent (PermPol-PES)22; two studies randomising BioPol-BES versus PermPol-SES29,33; two studies randomising BioPol-BES versus PermPol-PES15,16; and finally one study randomising BioPol-BES versus bare metal stent19.
Clinical endpoints at 30-days follow-up (Table 2)
TLR: Among the studied population, the incidence of TLR at 30 days was 0.4% in the BioPol-SES, 0.7% in the BioPol-PES and 1.4% in the BioPol-BES. These incidences were statistically significantly different (overall p-value=0.01); the three pair-wise comparisons were; OR=3.4, 95%CI=1.3-9.6, p=0.006 for BioPol-BES vs. BioPol-SES, OR=1.7, 95%CI=0.6-5.1, p=0.3 for BioPol-PES vs. BioPol-SES and OR=2.0, 95%CI=0.9-4.7, p=0.08 for BioPol-BES vs. BioPol-PES.
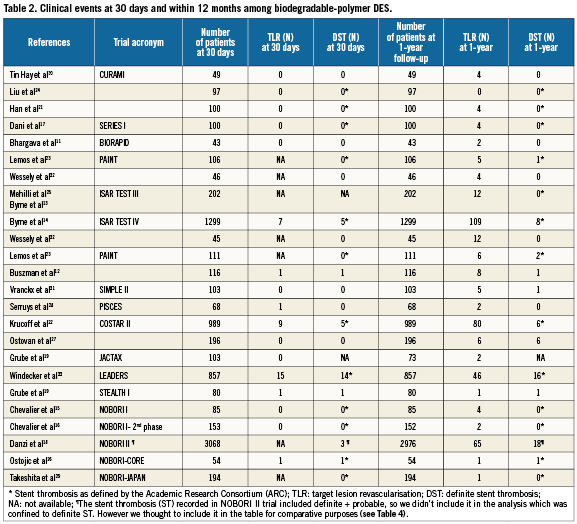
DST: The incidence of DST at 30 days was 0.2% in the BioPol-SES, 0.3% in the BioPol-PES and 0.9 % in the BioPol-BES. The overall test on equality of these incidences showed a trend towards statistical significance (p-value=0.06); the three pair-wise comparisons were; OR=3.9, 95%CI=1.1-14.0, p=0.04 for BioPol-SES and BioPol-BES, OR=1.4, 95%CI=0.3-6,0, p=0.6 for BioPol-SES vs. BioPol-PES and OR=0.4, 95%CI=0.1-1.2, p=0.09 for BioPol-BES vs. BioPol-PES.
Clinical endpoints at one-year follow-up (Table 3)
TLR: Over a follow-up period up to 12 months, the incidence of TLR among the studied population was 4.9% in the BioPol-SES, 6.1% in the BioPol-PES and 2.3% in the BioPol-BES. These incidences varied significantly among the different stents (overall p-value=0.03). There was almost three times higher risk of TLR in the BioPol-PES compared to the BioPol-BES (OR=2.8, 95%CI=1.3-6.0, p=0.01), and twice higher risk of TLR in the BioPol-SES compared to BioPol-BES (OR=2.2, 95%CI=0.2-1.0, p=0.04), and no significant difference in the risk ratio of BioPol-SES vs. BioPol-PES (OR=1.3, 95%CI=0.6-2.6, p=0.5).
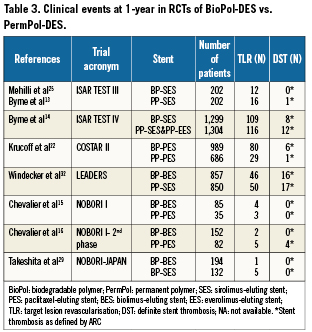
DST: The incidence of DST at one year follow-up was 0.3% in BioPol-SES, 1% in the BioPol-PES and 0.8% in the BioPol-BES. The pooled odds-ratio was not significant among the different stent comparisons (overall p-value=0.2).
Clinical endpoints at one-year in randomised clinical trials of BioPol-DES vs. PermPol-DES
In another analysis, in which clinical endpoints were assessed in studies comparing BioPol-DES with PermPol-DES in a randomised manner (seven randomised controlled studies)13-16,22,25,29,33, it was observed that risk of developing TLR at one year follow-up was not significantly different in PermPol-DES compared to BioPol-DES (OR=0.8, 95% CI=0.5-1.4, p=0.5) (Figure 2). Similarly, the one year risk of DST was not significantly different in PermPol-DES compared to BioPol-DES (OR=0.7, 95% CI=-0.2-2.4, p=0.5) (Figure 3).
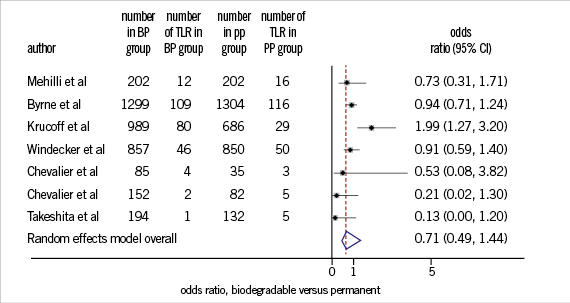
Figure 2. One year TLR in BP-DES vs. PP-DES. TLR: target lesion revascularisation; BP: biodegradable polymer; PP: permanent polymer; DES: drug-eluting stents
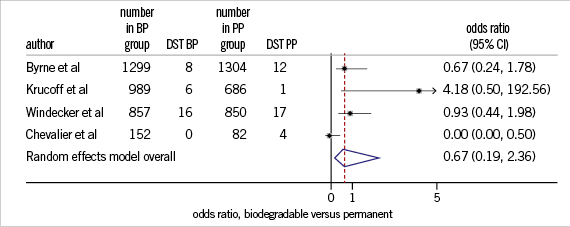
Figure 3. One-year DST in BP-DES vs. PP-DES. DST: definite stent thrombosis; BP: biodegradable polymer; PP: permanent polymer; DES:drug-eluting stents
Randomised clinical trials quality assessment
Each of the RCTs comparing BioPol-DES with PermPol-DES had five or more questions answered with “yes” when assessed with the Delphi list. Therefore, all seven RCTs were considered to have alow risk of introducing bias in the assessment of TLR or DST in BioPol-DES vs. PermPol-DES.
Discussion
Three types of biodegradable polymer based DES were analysed in our study; sirolimus, paclitaxel and biolimus A9.
Rapamycin (sirolimus), is a macrolide with cytostatic properties that blocks progression from G1 to S in the cell cycle and inhibits thus the vascular smooth muscle cell migration and proliferation34.
Biolimus A9 is an analogue of rapamycin that binds to FK binding protein 12 and subsequently to the mammalian target of rapamycin. The formed complex inhibits smooth muscle cells proliferation by blocking the cell cycle progression between the G1 and S phase. The main difference between biolimus A9 and rapamycin is replacement of hydrogen by alkoxy-alkyl group at 40-O position, increasing its lipophilicity which is expected to optimise the drug distribution16. Two similar types of biolimus A9 eluting stents were tested in previous studies, The BioMatrix® (Biosensors Interventional Technologies Pte Ltd, Singapore) and The NOBORI® (TERUMO Europe NV, Leuven, Belgium).
Paclitaxel inhibits vascular smooth muscle cell migration and proliferation mainly as a result of binding to and stabilising cellular microtubules34,35.
All stents were coated with a biodegradable polylactic acid (PLA) or polylactic-co-glycolic acid (PLGA) polymer7. In principle, after drug delivery and subsequent complete polymer degradation, only the biologically inert bare-metal platform remains.
To our knowledge this is the first meta-analysis that compares the performance of different DES with biodegradable polymers in a large cohort of patients with similar inclusion criteria, aiming to judge the individual drug performance without the influence of permanent polymer. The key findings were that: a) the risk of TLR and DST were highest in the BioPol-BES group within short term follow-up (30 days); b) The risk of TLR at one year follow-up was three times higher in the BioPol-PES and twice higher in the BioPol-SES when compared to the BioPol-BES; c) There was no significant difference in the one year risk of DST between the studied groups, however we could still observe a higher incidence of ST in BioPol-PES compared to BioPol-SES (1% vs. 0.3%).
This meta-analysis is based on comparisons between studies. Aconsequence of this is that the results are more amenable to risk of bias than most meta-analyses, which are based on comparisons of meta-analysed data randomised within studies. Thus our analysis yields valid results only under the assumption that, on the average, throughout these different studies the patient populations are not systematically different, though they are treated with different types of biodegradable polymer DES. In our view, this assumption is likely to be fulfilled since the inclusion and exclusion criteria were very comparable and were not dissimilar between groups in these various studies with different types of stent.
In an additional analysis performed in randomised trials only, we found that the 1-year risks of TLR and DST were not significantly different between BioPol-DES and PermPol-DES.
Long-term follow up results (>2years) are not yet fully available for the majority of biodegradable polymer DES and therefore we did not perform a long-term analysis.
Early stent thrombosis and target lesion revascularisation (TLR)
One of the interesting findings in this study was the significantly higher incidence of early stent thrombosis (EST), within 30 days, in the BioPol-BES group, and the subsequent higher incidence of 30 days-TLR compared to BioPol-SES and BioPol-PES.
These results should be interpreted cautiously, especially in the consideration that polymer or drug related stent thrombosis tends to present more likely as a mid- or late- term event, and that most of the early thrombotic events which have occurred in the BioPol-BES group were encountered in the LEADERS trial33, which involved adiversity of complex lesions. However, although the ISAR TEST-IV trial14 which tested BioPol-SES had similar inclusion criteria and diverse complex lesions, it resulted in less early thrombotic events. Moreover, when comparing the LEADERS33 and NOBORI-218 clinical trials, the two leading “all comers” biolimus A9 trials, we could observe that the incidence of EST was obviously different between both trials (1.6% vs. 0.1% respectively, Table 2).
It is still to early to adopt the hypothesis that a more intense antiplatelet regimen should be adopted in patients receiving BioPol-BES. Probably a more dedicated pharmacokinetic study, that addresses the issue of biolimus A9 tissue distribution and polymer degradation rates in different settings such as acute coronary syndromes and complex coronary lesions, would shed further light on this issue.
Target lesion revascularisation (TLR) at mid-term follow-up (1-year)
From this study it was concluded that the incidence of TLR was significantly lower in the BioPol-BES compared to both BioPol-SES and BioPol-PES. This goes along with the results of the NOBORI series of clinical trials15,16,26,29. A subanalysis of the LEADERS trial compared outcomes at one and two years36 in BioPol-BES vs. PermPol-SES patients, stratified according to the SYNTAX score37 tertiles. The authors showed that BES offered significant clinical benefit over SES among patients with high SYNTAX scores, among which was significantly less TLR at one year, with a strong trend at two-year follow-up. Recently, the three-year follow-up of the LEADERS trial has been announced, showing the sustained benefit of BES over SES in patients with high SYNTAX score and among patients with STEMI38. In view of these long-term results, it would be advisable to use BioPol-BES among patients with high-risk lesions.
Biolimus A9 possesses enhanced anti-inflammatory and antiproliferative activity with an improved pharmacokinetic profile. Unlike currently approved drug-eluting stents utilising drugs originally developed for other indications, biolimus A9 has specifically been developed for local delivery to coronary arteries39. Biolimus A9 is a novel rapamycin derivative that, like sirolimus, inhibits smooth muscle cell proliferation via binding to the FK-binding protein and subsequent inhibition of the mammalian target of rapamycin (mTOR)40-42.
The newly developed biolimus A9 eluting stents; Nobori® and BioMatrix® share several unique features. The most important are biodegradable polymer carrier (polylactic acid), and coating only on the abluminal stent surface. The later feature allows direct release of biolimus A9 into the vessel wall and, enhanced by its high lipophilicity (~10-fold higher than sirolimus), fast uptake by the surrounding tissue15,16,33,43.
It has been previously reported that sirolimus and paclitaxel drug-eluting stents were associated with paradoxical coronary vasoconstriction up to 12 months after implantation44-47. This observation may be attributable to delayed endothelialisation caused by the drug and/or endothelial dysfunction caused by polymer-induced inflammation or hypersensitivity reaction. On the contrary, in a recent study, it has been shown that biolimus A9 eluting stents are associated with better preserved endothelial function in coronary arteries compared to the first generation DES, which could be partly explained by the better drug release kinetics48.
Animal studies showed that after BES implantation, the tissue concentration of the drug in segments 5mm proximal and distal to the stent edges is almost non-measurable43. In addition, SES and BES have different drug release kinetics: total drug content is released from the SES within 60 days with more than 60% released shortly after stent implantation49, versus a small initial burst and sustained simultaneous drug release and polymer degradation taking place over 6-9 months in the BES43, exposing the surrounding tissue at any given time to a lower amount of drug.
Definite stent thrombosis (DST) at mid-term follow-up (1-year)
Because durable polymers have been held responsible for some of the thrombotic events that are assumed to occur as a result of polymer-mediated inflammatory reaction and delayed endothelialisation, it was expected that degradation of the polymer will improve arterial healing and thus negate this adverse outcome. This leaves us with the assumption that a higher incidence of thrombotic events, if any, would be a “drug only” effect.
In this meta-analysis, the risk of DST was not significantly different between the different BioPol-DES at one year follow-up; however we could still observe that the incidence of ST was numerically three times lower in SES vs. PES (0.3% vs. 1% respectively). Previous data, comparing first generation DES, have shown that there is higher risk of ST in PES compared to SES3,50,51.
Biodegradable-polymer Biolimus A9 stents (BioMatrix® vs. NOBORI®)
Despite using the same biodegradable polymer (polylactic acid) coated on the abluminal stent surface, recent pharmacokinetic studies of the two main biolimus A9 stents; Biomatrix®52 and NOBORI®43, have shown that there is an obvious difference between the pharmacokinetics of the the two main biolimus A9 stents, which may be an explanation for the more favourable clinical outcomes encountered with the latter (Table 4).
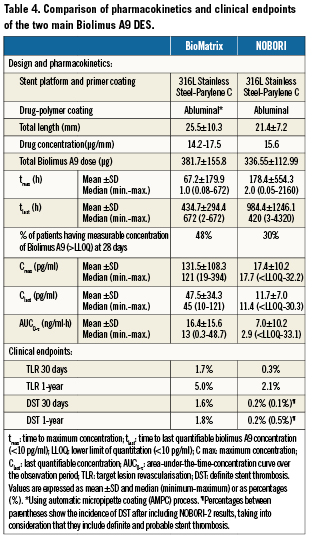
The NOBORI® stent design and coating process differ from that of the BioMatrix® stent. The maximum biolimus A9 concentrations in blood with a median of 18pg/mL (range
In addition to drug distribution and elimination, biolimus A9 pharmacokinetics are significantly affected by the polymer degradation rate which in turn is influenced by the state of the vessel. Ina vessel with inflammation, the pH is expected to be low which will accelerate the degradation of the polymer material and the subsequent drug release into the vessel wall7.
Clinical outcomes in randomised clinical trials of BioPol-DES vs. PermPol-DES
In the sub-analysis confined to randomised clinical trials comparing BioPol-DES and PermPol-DES, it was found that the risks of TLR or late DST at 1-year were not significantly different between both groups.
Unexpectedly, it was found that the presence of biodegradable polymeric coating did not influence the risk of stent thrombosis in DES at 1-year follow-up. Lately there is an increasing awareness about the biocompatibility of permanent polymer implants and their potential role in contributing to stent thrombosis. Many animal studies have shown that a hypersensitivity reaction may occur as a result of polymer induced inflammation7,53. Given these issues, more focus has been placed upon developing biodegradable polymers which degrade over time.
In a previous study, van der Giessen et al has demonstrated that biodegradable polymers may induce marked inflammatory reactions in the porcine coronary arteries, and that this may be attributable to the combination of the parent polymer compound, and biodegradation products54. Moreover, there are several factors which influence the velocity of degradation, either by accelerating or slowing it. Accordingly, It is has be stated that a balance between drug release kinetics, the rate of degradation of the polymer and the degradation products are essential for the success of bio-erodible stent systems in coronary vasculature7. New families of biodegradable polymers have been tested recently in animal studies implemented by Lockwood and colleagues55 which yield fewer acidic by-products than standard biodegradable polymers (PLGA, PLA) used in the currently available BioPol-DES, thus making it well-tolerated in vivo by providing a better degradation rate and biocompatibility profile.
In an attempt to overcome the problems encountered with polymers or their degradation products, “polymer-free” drug-eluting stents have evolved and have been proven to be safe in some clinical studies13,56, they were even associated with less late lumen loss compared to biodegradable polymer and permanent polymer stents in one study13. In a recent animal study, polymer-free biolimus A9 coated stents demonstrated more sustained intimal inhibition, improved healing and reduced inflammation compared with the polymer coated sirolimus eluting Cypher® stent57. One would expect that a DES without polymer will release the drug in a relatively short amount of time, resulting in relatively high systemic and tissue peak concentrations, however the superior pharmacokinetics and long half life of biolimus A9 in the target tissues (biolimus A9 was present in the coronary tissue until 180 days after stent implantation), made it a suitable drug for coating on non-polymer stents.
Limitations
To maximise the utilisation of all available data, we included abstract presentations that have not been subjected to as much peer review and scrutiny as with full papers, and may not be as of high a quality; however, we felt that this was necessary for two reasons: Including abstracts served as an additional tool to avoid any potential publication bias. The second reason is that the magnitude of treatment effect can be overestimated by analysing only the published data58.
In our study only DST was used in the analysis, which may underestimate the true incidence of stent thrombosis among the studied stents. We preferred to use this endpoint rather than overall stent thrombosis since not all the included studies reported stent thrombosis as definite, probable or possible according to the ARC definition8. In those studies not using the ARC definition, their specific definition of stent thrombosis stated that it was angiographically documented, which matches definite stent thrombosis as defined by the ARC.
Conclusions
This meta-analysis, comparing different biodegradable-polymer DES, showed that the risk of early DST and subsequently TLR were highest in the BioPol-BES at 30 days follow-up, whereas at 1-year there was significantly less TLR in the BioPol-BES. We could observe a three times higher incidence of ST in BioPol-PES compared to BioPol-SES at 1-year. On comparing BioPol-DES and PermPol-DES in randomised clinical trials, there was no significant difference in the risk of either TLR or stent thrombosis at 1-year.
These results point to the fact that BioPol-DES do not necessarily perform better than PermPol-DES and that short-, mid- and long-term results are to be carefully judged separately for newly emerging BioPol-DES before they can become a new standard.
Conflict of interest statement
The authors have no conflict of interest to declare.