Abstract
Aims: Drug eluting stents (DES) are currently considered the gold standard for reducing restenosis of coronary artery lesions. Owing to their effect on the healing process, DES use requires mandatory prolonged dual antiplatelet therapy (DAT). The endothelial progenitor cell (EPC) capture stent, attracting circulating EPCs, promotes vascular healing and allows a short post-procedural period of DAT. The aim of the present study was to evaluate the short and long term clinical outcomes of the use of the Genos R stent™ in a selected high risk population with “no option” for DES.
Methods and results: From December 2005 to October 2008, 61 high risk patients with clear contraindications to a prolonged period of DAT who underwent PCI with EPC capture stent implantation in our institution were prospectively selected and analysed. Technical success rate was 100%. Procedural success rate was 95.1%. After two years, major adverse cardiovascular events (MACE) free survival was 80.6%. According to the Academic Research Consortium definitions, cardiac death occurred in 1.6% of patients, and re-infarction, target lesion revascularisation (TLR), and target vessel revascularisation (TVR) occurred in 6.6%, 9.8%, and 11.5% of patients, respectively. Definite stent thrombosis occurred in one patient (specifically at 0 days). In patients who underwent surgery, no post-procedural MACE and no stent thrombosis were recorded.
Conclusions: EPC capture stent implantation in high-risk patients with no option for DES seems encouraging, with satisfactory clinical outcomes both at short and at long term follow-up.
Introduction
Drug eluting stents (DES) significantly reduce neointimal hyperplasia1,2. The anti-proliferative capability of DES, however, negatively affects the natural vascular healing process, inhibiting the activity of mature endothelial cells, promoting the senescence of endothelial progenitor cells (EPC), impairing the EPC functional activity and inducing endothelial dysfunction3,4. In patients treated with DES, prolonged dual antiplatelet therapy is mandatory to reduce the risk of adverse events related to stent thrombosis (ST)5. The EPC capture stent (Genous R stent™, OrbusNeich, Fort Lauderdale, FL, USA) is a bioengineered stainless steel, dual-helix, modular stent coated with murine monoclonal antihuman CD34 antibodies designed to attract circulating EPCs, establish a functional endothelial layer and therefore accelerate vascular healing. Early surface endothelialisation, reducing the risk of ST, allows a short post-procedural period of DAT6,7.
The aim of the present study was to evaluate the efficacy and safety of the use of EPC capture stent in a prospective cohort of high risk patients with underlining clinical conditions that prevented the use of DES.
Methods
Consecutive patients who underwent PCI with EPC capture stent implantation from December 2005 to October 2008 in our institution were prospectively selected and analysed. Patients were eligible for inclusion if they were defined as high-risk and had a clear contraindication to a prolonged period of DAT, condition precluding DES implantation.
Exclusion criteria were significant left main disease and cardiogenic shock. No other predefined clinical inclusion or exclusion criteria were considered.
Primary outcome measures investigated were the occurrences of death, myocardial infarction (MI), target vessel revascularisation (TVR), target lesion revascularisation (TLR), and major adverse cardiac events (MACE) defined as a non-hierarchical composite of all cause death, nonfatal MI, or repeat revascularisation during hospital stay and at long-term follow-up.
Definitions
A high-risk condition was defined as meeting two or more of the following criteria: diabetes, acute coronary syndrome, heart failure, proximal vessel disease, multivessel disease, B2/C type lesion, bifurcating lesion, long lesion. Patients were defined as “no option” for DES in presence of: a) known aspirin hypersensitivity or allergy; b) pathologies requiring surgical intervention within 60 days off the procedure; c) high haemorrhagic risk. High haemorrhagic risk was defined as meeting one of the following criteria: known bleeding disorders, stroke during the preceding six months, neoplasms, recent major trauma/surgery, active peptic ulcer, infective endocarditis, advanced liver disease.
Technical success was defined as successful deployment of a stent(s) in the target lesion. Procedural success was defined as lesion revascularisation with ≤30% residual diameter stenosis by quantitative coronary angiography, without major procedural or post-procedural adverse events (death, myocardial infarction, emergency target vessel revascularisation or acute stent thrombosis). Death was classified as either cardiac (CD) or non-cardiac, according to the Academic Research Consortium (ARC) definition8. Deaths that could not be classified were considered cardiac. TLR was defined as any repeat percutaneous intervention of the target lesion or other complication of the target lesion. The target lesion was defined as the treated segment from 5 mm proximal to the stent to 5 mm distal to the stent. TVR was defined as any repeat PCI of any segment of the target vessel, defined as the entire major coronary vessel proximal and distal to the target lesion, including upstream and downstream branches and the target lesion itself. Myocardial infarction was defined according to the ARC definitions8. MACE were defined as the occurrence of cardiac death (CD), non-fatal myocardial infarction (MI) or TVR during the follow-up period. Definite, probable and possible stent thromboses (ST) were determined according to the ARC definitions. Stent thrombosis was defined as acute, sub-acute, late and very late if the event occurred within 24 hours, 30 days, <1 year or >1 year respectively, after the procedure.
Procedures, medications and follow-up
All PCIs were performed according to current guidelines. Stenting strategy, use of periprocedural glycoprotein IIb/IIIa inhibitors, route of arterial access, predilation devices, intravascular ultrasound guidance and prophylactic intra-aortic balloon pump use was at the discretion of the operator. All patients were pretreated with aspirin (or indobufen 150 mg twice daily in case of aspirin hypersensitivity/allergy) and clopidogrel (75 mg/day for three days prior to the procedure or 300-mg loading dose) and low-molecular-weight or unfractioned heparin (administered in the cathlab, before guidewire insertion). Heparin was titrated to maintain an activated clotting time >250 s. After the procedure, all patients were prescribed lifelong aspirin (indobufen or clopidogrel alone in case of aspirin hypersensitivity/allergy) and a short (15 to 30 days) dual antiplatelet therapy (DAT) of aspirin 100 mg/day (or indobufen 150 mg twice daily) and 75 mg/day clopidogrel. In particular, six patients (10%) received DAT for 15 days, six (10%) patients for 21 days and 49 patients (80%) for 30 days. Repeated revascularisation was only performed if there was either symptom recurrence or inducible ischaemia related to the treated lesion. Immediate statin therapy in the form of atorvastatin 20 mg was started after the procedure.
A follow-up visit or telephone interview was scheduled at 30 days, six months, one year, and then yearly. Civil registries were queried in case of death, to determine whether it was or not a cardiac death. All repeat interventions and re-hospitalisation were prospectively collected during follow-up and entered into a dedicated database. Angiographic follow-up was not scheduled per protocol, but was performed only in case of positive stress test or recurrence of symptoms.
Statistic analysis
Variables with normal distribution were analysed using parametric tests while variables with a non-normal distribution were analysed with non-parametric tests. Continuous variables are expressed as mean ±SD or median±SD. Categorical variables are expressed as counts and percentages. All statistical tests were two-tailed. MACE are reported hierarchically. All analyses were performed using SPSS version 12 statistical software (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered significant.
Results
Baseline clinical characteristics
Baseline clinical characteristics are shown in Tables 1 and 2.
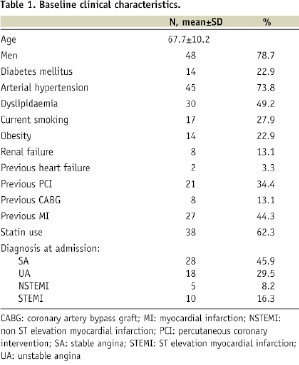
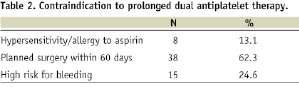
Mean age was 67.7±10.2 years, 48 patients (78.7%) were male and 14 (22.9%) were diabetics. Almost one-half of the patients (27, 45%) had a prior history of acute myocardial infarction, 21 (35%) had had a previous PCI, and eight (13.1%) had previously undergone CABG. The most common admission diagnosis was stable angina (28, 45.9%), followed by unstable angina (18, 29.5%), STEMI (10, 16.4%), and NSTEMI (5, 4.2%). Mean ejection fraction was 52±13 % and mean logistic EuroSCORE was 6.8±2.8%. “No option” for DES was due to known aspirin hypersensitivity or allergy in eight (13.1%) patients, planned surgical intervention within 60 days in 38 (62.3%) and high haemorrhagic risk in 15 (24.6%) patients.
Angiographic and procedural characteristics
Procedural characteristics are summarised in Table 3.
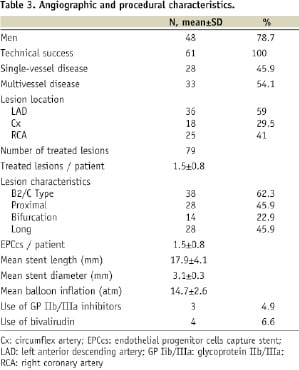
Briefly, one-half of the patients (54.1%) had multivessel disease. The most common lesion location was LAD (45.6%) followed by RCA (31.6%) and Cx (22.8%). Overall, a total of 79 lesions were treated and the stent per patient ratio was 1.5±0.8. B2/C type lesions were present in 62.3% of patients, proximal lesions in 45.9%, bifurcating lesions in 23% and long lesions in 45.9%. Mean stent length was 17.9±4.1 mm (range 9-23 mm), mean stent diameter was 3.1±0.3 mm (range 2.75-3.5 mm), and mean atmosphere inflation was 14.7±2.6 atm (range 10-24 atm). Elective bivalirudin administration was performed in four (6.6%) patients while bail-out glycoproteins IIb/IIIa infusion was performed in three (4.9%) patients.
Follow-up clinical outcomes
Follow-up outcomes are summarised in Table 4.
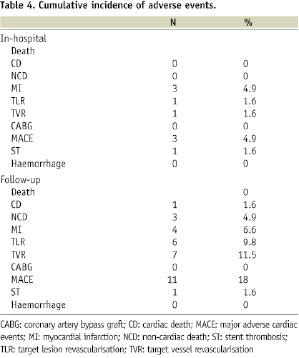
Technical success was achieved in all cases. No patient died during the hospital stay, one patient (1.6%) showed acute stent thrombosis 20 hours after the procedure and required a re-PCI. No patient required emergent bypass surgery. Three patients (4.9%, including the patient with acute ST) had post-procedural MI. Procedural success rate was 95.1%.
At follow-up (mean: 14,8±9 months), the cumulative incidence of cardiac death was (1.6%) and the incidence of MI, TLR and TVR were 6.6%, 9.8% and 11.5% respectively. A MACE occurred in 18% of patients. No cases of subacute, late or very late stent thrombosis were detected and no patient required bypass surgery. The Kaplan-Meyer analysis showed a MACE-free survival of 80.6% both at 12 and 24 months (Figure 1).
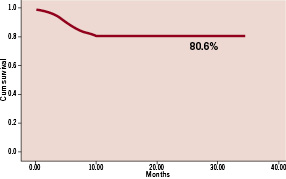
Figure 1. MACE free survival rate (80.6% both at 12 and 24 months).
Due to the small sample population, no independent predictors of adverse events were identified at the multivariate analysis. Bivariate analysis showed a significant correlation between STEMI at clinical presentation and MI at follow-up (p=0.017), and between TLR and B2/C type lesions (p=0.04), long lesions (p=0.032) and multivessel PCI (p=0.046). In the surgical subgroup, the mean time of surgery after PCI was 51±11.6 days, without the occurrence of any major cardiac event, including ST during or after the operative period.
Discussion
The main findings of this report are the following: a) EPC capture stent use in a selected population of high-risk patients with no-option for DES resulted in satisfactory clinical outcomes both at short and long term follow-up; b) despite the short period of DAT, no sub-acute, late or very late ST occurred; c) in patients who underwent surgical treatment short time after PCI, no perioperative MACE or thrombotic event occurred; d) no major or minor bleeding occurred.
DES are currently the gold standard for the treatment of significant coronary artery stenosis, due to their proven efficacy to reduce neointimal hyperplasia and clinical adverse events rate9. However, in patients with underlining clinical conditions that contraindicate the mandatory prolonged double antiplatelet therapy, the use of DES must be avoided10. In these particular cases, the use of a stent that accelerates the natural healing process and allows a short post-procedural period of DAT could be a safe and effective strategy. A first-in-man study of the EPC capture stent demonstrated the safety and feasibility of this device for the treatment of de novo coronary artery disease6 and the preliminary data from the HEALING II7 showed low MACE and low stent thrombosis rate in stable patients undergoing elective PCI.
For the first time, the present results indicate that EPC capture stent implantation in patients with known hypersensitivity / allergy to aspirin, coexisting pathologies requiring surgical intervention which cannot be deferred or underlying medical conditions resulting in an high risk of bleeding is feasible and effective overtime. Due to the specificity of the selected population, it is difficult to put into perspective the results of this study. Previous reports of DES use conducted in high risk populations showed similar incidences of MACE at long-term follow-up11-13 suggesting the EPC capture stent to be a possible alternative to DES in terms of efficacy.
One of the most important observations in this study is the lack of late and very late stent thrombosis in patients who received the EPC capture stent. The only thrombotic event observed was an acute stent thrombosis occurred 20 hours after the PCI procure in an 80 year old man with a EuroSCORE=8 and multivessel disease, who had an arrhythmic complication (pulseless electrical activity) during the procedure that required cardiopulmonary resuscitation manoeuvres. This is of particular importance given the concern with increased rate of late stent thrombosis with DES14,15. The safety profile, even in this high-risk patient group, appears satisfying and comparable to that of DES16, both in patients who received regular DAT as well as in patients who, because of aspirin hypersensitivity / allergy, received a 30 days DAT of indobufen + clopidogrel.
The optimal timing for non-cardiac surgery after percutaneous coronary intervention with DES is not known yet, but recent evidence suggests that surgery should be deferred at least three months after PCI with DES implantation17. In fact, early non-cardiac surgery after coronary stent placement is associated with an increased risk of major adverse cardiac events, the majority of which are attributable to stent thrombosis18,19. Antiplatelet therapy interruption in the perioperative period seems to play a key role in the increase of adverse events, particularly in patients who received DES. In the surgical subgroup of the present study, the “healing strategy” with the use of EPC capture stent demonstrated to be safe and effective. All patients underwent surgery within 60 days of the procedure without the occurrence of any major cardiac event, including ST. None of the patients was on DAT at the time of the surgical intervention. Moreover, aspirin was discontinued in all the surgery patients and was substituted with low molecular weight heparin for the few days before and immediately after the surgery. This result seems to clinically confirm what has been previously demonstrated in pre-clinical studies, where at one hour after deployment the EPC capture stent showed a >90% cell coverage and a rapid establishment of a functional endothelial layer20.
The need of a shorter period of DAT, together with a low risk for ST, makes the EPC capture stent an attractive device that can help physicians to safely manage this niche of high-risk patients without any slow down that could severely impair their prognosis.
A short DAT period implies as well a lower risk of haemorrhagic complications. Bleeding complications due to prolonged DAT actually are quite common. In a recent study conducted in a large population of patients treated with DES, bleeding events occurred in 32.4% of patients (85.7% were nuisance, 13.6% internal, 0.7% alarming) and the rate of clopidogrel discontinuation as a result of bleeding in the nuisance bleeding group was 11.1%21. Early clopidogrel cessation is detrimental to longer-term prognosis and significantly associated with stent thrombosis16,22. From the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) registry, thienopyridine discontinuation was associated with greater mortality and of hospitalisation rates at follow-up23. Currently, there are no guidelines and little evidence on how best to manage patients who are at high risk of bleeding. In our series, no major or minor bleeding was recorded both during the hospital stay and at follow-up, suggesting EPC capture stents as a safe and effective alternative to BMS in patients who cannot be prescribed prolonged DAT because of the high risk of bleeding complications.
Limitations
The present study was designed as a prospective single centre registry, and therefore lacks randomisation and intention-to-treat data. Sample size was relatively small and events adjudication was not blinded. However, given the lack of large studies on EPC capture stent implantation in this peculiar subset of patients and especially of any study reporting on long-term outcomes, this study, despite its inherent limitations in internal and external validities, adds an important piece of information about the use of this novel device.