A visionary idea, way ahead of its time
In this current issue of EuroIntervention there are three articles evaluating the endothelial progenitor cell capture stent published back-to-back. This is remarkable for many reasons, and deserves some commentary.
A little history
Michael Kutryk is the name of the interventional cardiologist and scientist who had this dream. As early as the late 90s, Michael had the vision that it could be most desirable to heal strut coverage specifically with functional endothelial cells in order to improve the biocompatibility of metallic stents. The concept of designing a “pro-healing” device with accelerated restoration of endothelial continuity was born at least at first in the mind of its inventor, but was quickly supported by bench and preclinical experiments1. By this time, Kutryk had left Canada to join the group at the Thoraxcenter in Rotterdam as a post-doctoral fellow (Figure 1).

Figure 1. Dr. Michael Kutryk.
During this period, Patrick Serruys was experimenting with local drug delivery with sweating balloons and, needless to say, he was immediately taken with Kutryk’s idea. Perhaps somewhat naively, they hypothesised that endothelial progenitor cell capture could prevent at one and the same time both thrombosis and restenosis2. It should be remembered that in those early days of stenting, the issues were immediate stent thrombosis and early restenosis, at rates of 20% each! Nobody at that time was anticipating that late thrombotic events would plague the future anti-restenosis solutions, initially vascular brachytherapy and now drug-elution.
The Genous technology invented by Kutryk and embraced by Serruys was developed and supported by Orbus Medical Technologies, and later acquired by Neich, an Asian-based company to form Orbus-Neich. Major research and development efforts eventually led to the First-In-Man trial that was published in 20053. The illustration (Figure 2) depicting the principles of the therapy was indeed very convincing, to the point that it was eventually included in the patient informed consent document for further studies.
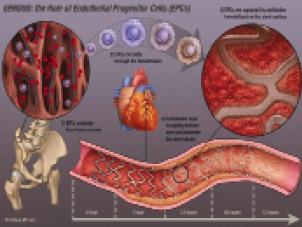
Figure 2. Schematic illustration of the principles of action of the endothelial progenitor capturing stent.
It shows how the stent is covered with a biocompatible matrix to which murine, monoclonal, anti-human CD34+ antibodies are covalently attached. The antibody is specific to the surface antigens, present on circulating endothelial progenitor cells creating an immuno-affinity surface for preferentially capturing these circulating cells. CE mark approval was obtained on August 11, 2005. The HEALING clinical trial program was designed and indeed completed, and publications appeared at an increasing pace. The device also underwent a number of technical iterations including the use of a cobalt-chromium stent platform, to replace the bio-engineered R-stent.
Present contributions
Silber et al4 are reporting the 12 month outcome of the 4,939 patient e-HEALING, world-wide, post-marketing registry. Target vessel failure was 8.4% and clinically-driven target lesion revascularisation was 5.7%, figures that are comparable to drug-eluting stents in similarly organised registries. Definite and probable stent thrombosis rates were 1.1% at one year. Although dual antiplatelet therapy is recommended for only one month, 83, 59 and 34% of patients were on combined aspirin and thienopyridine treatment at 1, 6 and 12 months, respectively.
Scacciatella et al5 present a single centre registry report on 2-year outcomes in 61 patients at high risk for restenosis but who cannot receive drug-eluting stents. The reasons for this were: planned surgery within two months, high bleeding risk or allergy to aspirin. MACE-free survival rate was 80.6% at one and two years. Target lesion revascularisation was 11.5%. A single case of stent thrombosis occurred shortly after the procedure. Duration of dual antiplatelet therapy was between 15 and 30 days.
Martin-Yuste et al6 report on another single centre registry. PCI with the Genous stent was performed in 78 patients with high comorbidity who required chronic anticoagulation. Dual antiplatelet therapy was added to anti-vitamin K treatment for one month. After that, aspirin was continued together with anticoagulant drug. Reasons for anticoagulation were mostly atrial fibrillation, mechanical valve prosthesis, cardiomyopathy or prior emboli. MACE included 12% mortality (six cardiac and four non-cardiac) and two strokes. Target lesion revascularisation was 7.8%. No acute myocardial infarction or stent thromboses occurred up to 14 months.
What is the clinical role for the endothelial progenitor capturing stent?
The initial disruptive promise of the pro-healing concept was that both restenosis and thrombosis could be prevented.
Although low repeat revascularisation rates have been observed in lesion and patient subsets with low propensity for restenosis, rates have been higher than achievable with drug-eluting stents in more complex situations. This led to the design of a multicentre, randomised, controlled, 2-armed TRIAS study with target lesion failure as a primary endpoint7. In 1,260 patients at low risk for restenosis, superiority of the endothelial progenitor capturing stent would be tested against a bare metal stent. In 1,300 patients at high risk for restenosis, non-inferiority of the endothelial progenitor capturing stent would be tested against a drug-eluting stent. The latter hypothesis was probed in a 193 patient pilot study that was recently reported8, with challenging results regarding the viability of the TRIAS concept. At one year, target vessel failure was 10.5% in the TAXUS versus 17.3% in the GENOUS group, a 6.8% risk difference due to higher need for repeat revascularisation. No stent thrombosis was seen with GENOUS versus four with TAXUS.
As to thrombo-resistance of the endothelial progenitor capturing stent, available data seem to be both consistent and promising, with low thrombosis rates in spite of shorter duration of dual antiplatelet therapy. It cannot be denied that the obligatory, long-term, stent-driven indications for dual antiplatelet therapy can complicate patient management in the presence of comorbidities, planned surgery and poor compliance to the point that the use of drug-eluting stents may become relatively contra-indicated9 see Table 35. In these situations, the use of the endothelial progenitor capturing GENOUS stent could be a useful alternative to both drug-eluting and bare-metal stents, as suggested by Scacciatella5 and Martin-Yuste6. However, one should recognise that establishing these indications will require additional, appropriately sized (large) prospective studies that consider both safety and efficacy endpoints. It is indeed likely that, compared to DES, more restenosis events will occur while attempting to prevent stent thrombosis and bleeding complications. The net clinical benefit will eventually establish the value of the endothelial progenitor capturing stent.
Of note, the reported studies have focused on elective procedures in rather stable patients. An open remaining question currently under investigation is whether GENOUS stents are effective in patients with unstable angina or STEMI.
Implications for development strategies of novel stents and vascular scaffolds
The saga of the endothelial progenitor capturing GENOUS stent is not over, yet it already illustrates some of the major difficulties that are associated with the development of novel endovascular therapies. First of all, the bar is set at a very high level. Requirements for regulatory approval and pre-clinical and clinical studies are massive. It has been a daunting challenge to attach the anti-human CD34 antibody to a sterile piece of metal that will be released with brutal force in a calcified vessel, all while keeping its exquisitely selective biologic properties. These and many other obstacles have been successfully crossed. Yet access to the competitive clinical scene does not necessarily imply success. Indeed the clinical results with the most recent generation DES are outstanding, and incremental improvements in outcome will necessarily be small, difficult to achieve and perhaps restricted to less optimally treatable subsets (acute infarction, diabetes, prior stent thrombosis).
Another lesson pertains to this unique concept of using autologous cell therapy for enhanced endovascular repair, i.e., biologics. The concept lives and dies by the number and quality of the endothelial progenitor cells that travel in the blood stream of each individual patient. Variability in response to the therapy will be inherent and enhancement factors may be needed in some patients10,11. Very early on, the GENOUS investigators have identified that healing was enhanced in patients receiving statins, an observation that was confirmed prospectively with high dose atorvastatin12. In specific clinical subsets such as diabetes, unstable angina, STEMI, chronic kidney disease, other factors that influence progenitor cell mobilisation, homing and functionality may be important and influence the interactions to implanted cell capturing stents. These complex biological responses will need to be further modulated in order to sufficiently enhance the efficacy of the therapy, thereby justifying its wider application.