Abstract
The present article focuses on recent innovations and possible future perspectives in the reperfusion treatment of ST-segment elevation myocardial infarction (STEMI). Among these, the shift from the femoral to the radial vascular access, the recent availability of bioresorbable coronary scaffolds, other innovative forms of stent specifically designed for STEMI patients, the use of cardioprotective strategies, as well as the possibility of including autologous bone marrow stem cell transplantation as part of the treatment of patients with STEMI are described and commented on as a glance into the future.
Vascular access
One of the most relevant changes introduced into the field of primary percutaneous coronary interventions (PCI) compared to the initial experiences relates to the vascular access. The use of the radial artery instead of the femoral artery in the specific setting of STEMI translates into a significant reduction in mortality mostly due to the reduction of bleeding, as investigated in the RIFLE and the RIVAL trials1,2. Of note, the significant reduction in mortality offered by the radial vascular access was particularly evident in patients presenting with STEMI compared with other forms of acute coronary syndrome (ACS). These data were subsequently confirmed by a large post hoc analysis3 and several additional investigations4-6.
Although the femoral artery may be preferred in some patients, such as those with a very weak radial pulse, or patients needing left ventricular haemodynamic support, or right ventricular pacing during the primary PCI, the radial access should probably be embraced as default for STEMI patients after adequate training in elective cases. It is worth mentioning that the use of dedicated femoral closure devices has not reduced the risk of complications associated with the femoral puncture7.
Mechanical reperfusion
Primary percutaneous coronary intervention is superior to fibrinolysis in that it reduces mortality, reinfarction and stroke, and also improves patency of the infarct-related artery, reducing the severity of the residual stenotic lesion, with an overall better left ventricular function8-10.
Furthermore, performing coronary angiography in STEMI allows immediate and accurate risk stratification based on the knowledge of the degree of the coronary artery disease and invasive haemodynamic parameters. Therefore, primary PCI is currently the recommended treatment of first choice for patients presenting with STEMI when performed in experienced centres and with limited time delay11. Nevertheless, fibrinolysis remains a first-line approach in many areas of the world where primary PCI is not an option, or at least is not “the first option available”12.
The optimisation of the social impact of reperfusion treatment will continue to expand such an approach in many areas of the world before a systematic access to well-trained cathlabs can become a reality, education and training of paramedics being the first step of this worldwide commitment.
Primary percutaneous coronary interventions
BALLOON-EXPANDABLE STENTS
Bare metal stent (BMS) implantation during primary PCI was initially associated with improved angiographic results and a decreased need for target vessel revascularisations13,14 compared with balloon angioplasty but failed to add a clear advantage in terms of mortality.
The introduction of first-generation drug-eluting stents (DES) brought a further reduction of restenosis with a subsequent lower rate of repeat revascularisations, but again without providing benefits in terms of mortality and reinfarction15, and the initial enthusiasm related to DES implantation in STEMI patients was later tempered by the observation of higher rates of late and very late stent thrombosis16.
The high thrombotic burden and vessel vasoconstriction present in the acute phase leading to stent undersizing and late malapposition in addition to polymer-related persistent inflammation and delayed vessel healing were identified as mechanisms involved in DES thrombosis16.
Second-generation DES with different drugs, biocompatible polymers, better stent designs and thinner struts, have overcome most of the limitations of first-generation DES. The use of second-generation everolimus-eluting stents (EES) with durable fluoropolymer in STEMI reduced target lesion and target vessel revascularisation compared to BMS (2.1% vs. 5.0%, p=0.003, and 3.7% vs. 6.8%, p=0.0077, respectively) at one-year follow-up with a lower incidence of stent thrombosis (definite stent thrombosis 0.5% vs. 1.9%; definite or probable stent thrombosis 0.9% vs. 2.5%, p=0.019)17.
Biolimus A9-eluting stents with biodegradable polymer tested in comparison with BMS reduced the occurrence of the composite of cardiac death, target vessel-related reinfarction, and ischaemia-driven target lesion revascularisation (4.3% vs. 8.7%, p=0.004) at one-year follow-up after primary PCI18, although the individual endpoints of death and reinfarction remained unchanged.
The introduction of next-generation DES with thinner struts and newer polymers, as well as the development of new stents dedicated to the treatment of STEMI patients may further improve the present clinical results.
SELF-EXPANDING STENTS
Self-expanding stents allowing low-pressure deployment were initially developed in an attempt to reduce vessel injury and neointimal response after stent implantation. In the specific setting of STEMI, the use of oversized balloons and high-pressure dilatation might trigger distal embolisation and no-reflow with a reduction of myocardial salvage. Therefore, the concept of gentle deployment and delayed self-expansion has recently been applied as an option for STEMI patients (Figure 1) and a thin-strut self-expanding, nitinol stent has recently been tested in patients presenting with acute myocardial infarction and compared with currently available balloon-expandable stents19, showing less strut malapposition at three days after implantation as assessed by optical coherence tomography, with similar clinical outcomes at six months between the two study arms. Larger dedicated clinical studies are currently needed to investigate further the clinical value of this new technology.
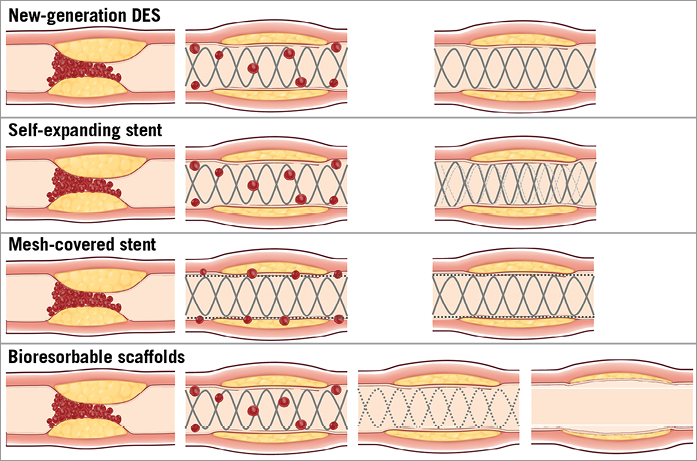
Figure 1. Different devices and mechanisms of revascularisation. New-generation DES provide a reduced incidence of target lesion revascularisation and a very low rate of stent thrombosis. Self-expanding stents with gentle deployment and delayed self-expansion could theoretically allow for a reduction in distal embolisation and less malapposition compared to balloon-expandable stents. Mesh-covered stents with the presence of a micronet mesh are intended to trap thrombotic material between the stent and the arterial wall, thus potentially reducing the risk of distal embolisation. The implantation of bioresorbable scaffolds provides revascularisation and, after bioresorption, restoration of vasomotor function, vessel compliance and cyclic strain in response to pulsatile flow, re-establishing the coronary physiology, potentially allowing for positive remodelling and late lumen enlargement.
MESH-COVERED STENT
Microvascular dysfunction after primary PCI is associated with increased infarct size and worse clinical outcomes20-23. Although many factors might promote such a phenomenon, distal embolisation of thrombotic debris from the culprit lesions during stent deployment seems to play a key role. Mechanical and pharmacological approaches have previously been tested to reduce the impact of thrombus embolisation, often with non-definitive results. However, despite the appealing concept of protecting the distal microcirculation with dedicated devices for distal or proximal capture of thromboembolic debris, randomised trials have failed to demonstrate a relevant clinical advantage of these strategies24,25.
Recently, a BMS covered with a polyethylene terephthalate micronet mesh was introduced in the clinical arena. The presence of a micronet mesh is intended to trap thrombotic material between the stent and the arterial wall, thus theoretically reducing the risk of distal embolisation (Figure 1). Several reports showed the safety and feasibility of this technology, and a randomised trial has been performed comparing the rate of complete (≥70%) ST-segment resolution measured at 60 to 90 min post-procedure, between mesh-covered stents and standard stents26.
This endpoint was significantly improved in patients treated with mesh-covered stents (57.8% vs. 44.7%; p=0.008), also with a superior rate of TIMI 3 flow (91.7% vs. 82.9%, p=0.006) with an equivalent myocardial blush grade 2 or 3 (83.9% vs. 84.7%, p=0.81) compared with standard stents. Although a mesh-covered stent in STEMI could have interesting implications especially in relation to the reduction of thrombus dislodgement and embolisation, its BMS nature and the presence of a permanent polyethylene micronet mesh suggest the need for a careful assessment of the restenosis rate.
BIORESORBABLE VASCULAR SCAFFOLDS (BVS)
Bioresorbable technologies represent a potential step forward in endovascular interventions. Their applicability in the STEMI setting (Figure 2) may provide additional benefit which could be maximised in young patients with long life expectancy. Acute coronary syndromes (ACS) and STEMI are often the first clinical manifestation of coronary artery disease (CAD) in young subjects, and the possibility of coronary artery treatment without permanent metallic implants could have important long-term implications.
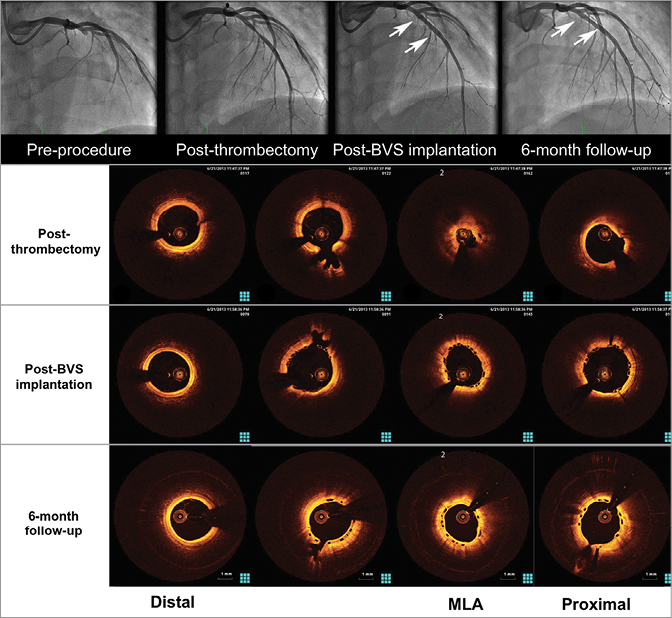
Figure 2. Treatment of an acute myocardial infarction with an Absorb BVS and six-month invasive follow-up. Upper panel: angiographic images at the index procedure and at midterm (six-month) follow-up showing optimal lumen patency. Lower panel: optical coherence images showing the presence of residual thrombus after thrombus aspiration but not after BVS implantation, supporting the concept of increased thrombus sequestration between the struts and the vessel wall due to the wider struts of the BVS (“Snowshoe” concept). At six-month follow-up the scaffold appears patent and fully covered with a thin layer of neointimal tissue.
Two studies have recently been published, reporting the feasibility and safety of Absorb BVS implantation specifically in STEMI patients27,28 along with a few other investigations with small series of STEMI and NSTEMI29.
Implantation of the Absorb BVS in STEMI patients yielded a high procedural success rate. Thrombus aspiration and lesion preparation were performed in a high percentage of patients as recommended because of the higher profile of the scaffold compared with standard stents. A theoretical concern regarding Absorb BVS implantation in STEMI is that a more extensive lesion preparation could result in increased distal embolisation and a higher risk of no-reflow. However, this effect was not reported in any of the above-mentioned preliminary evaluations. The deployment of a device with a larger strut width (Absorb BVS 157 µm) compared to metallic stents could induce a different pattern of thrombus dislodgement, with a percentage of vessel wall area paved by the BVS (scaffold/vessel ratio) reaching 26%, a higher value compared to metallic DES (i.e., EES provides a percentage stent/vessel ratio equal to 12%)30. This characteristic might be associated with an increased capacity for capturing thrombotic material behind the struts, reducing distal embolisation (“Snowshoe” concept)27. The perspective of vessel healing without a permanent metallic cage is of particular interest as it provides revascularisation and, after bioresorption, restoration of vasomotor function, vessel compliance and cyclic strain in response to pulsatile flow, re-establishing a normal coronary physiology, potentially allowing for positive remodelling and late lumen enlargement (Figure 1, Figure 3).
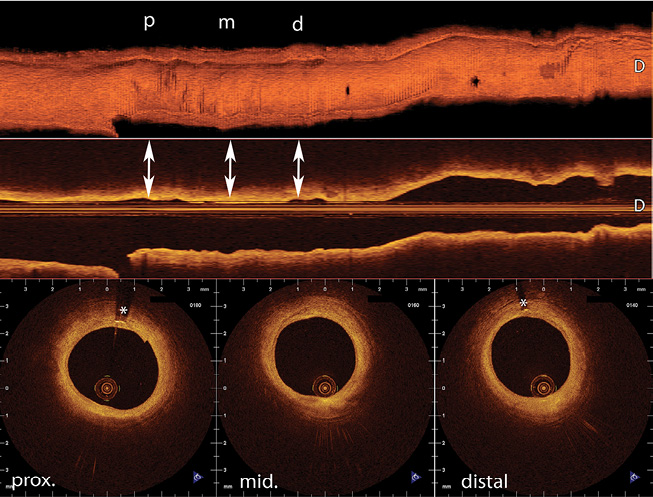
Figure 3. Three-dimensional (3D) reconstruction of a five-year follow-up OCT of a right coronary artery treated with a bioresorbable vascular scaffold (BVS) in a 59-year-old man, showing the vessel healing without a permanent metallic cage. The arrows show location of cross-sectional images (mid scaffold and distal edge). *Permanent markers at the edge of the BVS. 3D and longitudinal reconstruction created with CAAS Intravascular 1.1
Given the very limited data on the clinical use of the Absorb BVS in acute patients, several aspects need to be carefully evaluated, among them the incidence of scaffold thrombosis and the optimal antiplatelet strategy.
Cardioprotection: myocardial conditioning
ISCHAEMIC PRECONDITIONING (IPC)
Myocardial conditioning is a broad term used to describe the intriguing finding that brief, non-lethal episodes of myocardial ischaemia and reperfusion applied to an organ or tissue render the heart resistant to a subsequent episode of sustained, lethal ischaemia-reperfusion injury31 (Figure 4).
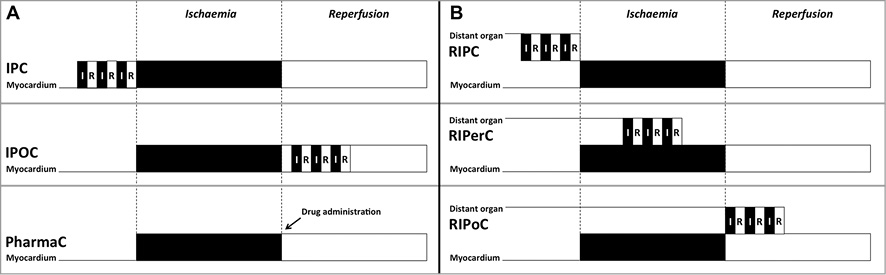
Figure 4. Timing of different conditioning strategies in relation to ischaemia and reperfusion periods. A) Ischaemic preconditioning (IPC) with episodes of myocardial ischaemia and reperfusion applied before reperfusion. Ischaemic post-conditioning (IPOC) with episodes of myocardial ischaemia and reperfusion applied after reperfusion. Pharmacological conditioning (PharmaC) with drugs intended to mimic the cardioprotective effects of myocardial conditioning. B) Remote ischaemic conditioning. Remote ischaemic preconditioning (RIPC) with episodes of ischaemia and reperfusion applied before reperfusion to an organ or tissue distant from the target organ requiring protection (myocardium). Remote ischaemic per-conditioning (RIPerC) with episodes of ischaemia and reperfusion applied during reperfusion to an organ or tissue distant from the myocardium. Remote ischaemic post-conditioning (RIPoC) with episodes of ischaemia and reperfusion applied after reperfusion to an organ or tissue distant from the myocardium.
However, the requirement to implement the IPC stimulus before the onset of ischaemia has restricted its clinical application to elective procedures in which the ischaemic episode can be anticipated, such as cardiac surgery.
Interestingly, the IPC stimulus was also found to be effective when applied to an organ or tissue distant from the target organ requiring protection (remote ischaemic conditioning). In fact, brief ischaemic episodes of the circumflex artery significantly reduced myocardial infarct size following sustained occlusion of the left anterior descending artery in dogs (i.e., remote intracardiac conditioning), and a 15 min period of small intestine or renal ischaemia was also capable of limiting infarct size (i.e., remote inter-organ conditioning)32,33. The observation that transient ischaemia and reperfusion of the limb could also elicit remote inter-organ conditioning facilitated the translation of this endogenous cardioprotective phenomenon into the clinical field34 and clinical trials evaluated the effect of remote ischaemic preconditioning in STEMI35. Bøtker et al showed that four five-minute arm cuff inflations and deflations applied by ambulance personnel during transport to the hospital were capable of increasing myocardial salvage and decreasing infarct size at one month35. Importantly, the greatest advantage was observed in patients presenting with occlusion of the left anterior descending artery, suggesting that patients most likely to benefit from this strategy are those sustaining large anterior infarctions. A subsequent trial has confirmed the protective effects of remote conditioning (three four-minute cycles of arm cuff inflations and deflations) in patients undergoing primary PCI on myocardial infarct size as measured by serum levels of troponin I36 and that the addition of morphine appeared to increase the beneficial effect of remote ischaemic conditioning. Most importantly, the follow-up study of the trial by Bøtker et al demonstrated that the reduction in infarct size obtained with remote ischaemic conditioning before hospital admission translated into a significant reduction in major adverse cardiovascular events at five years, which was principally driven by a reduction in mortality37.
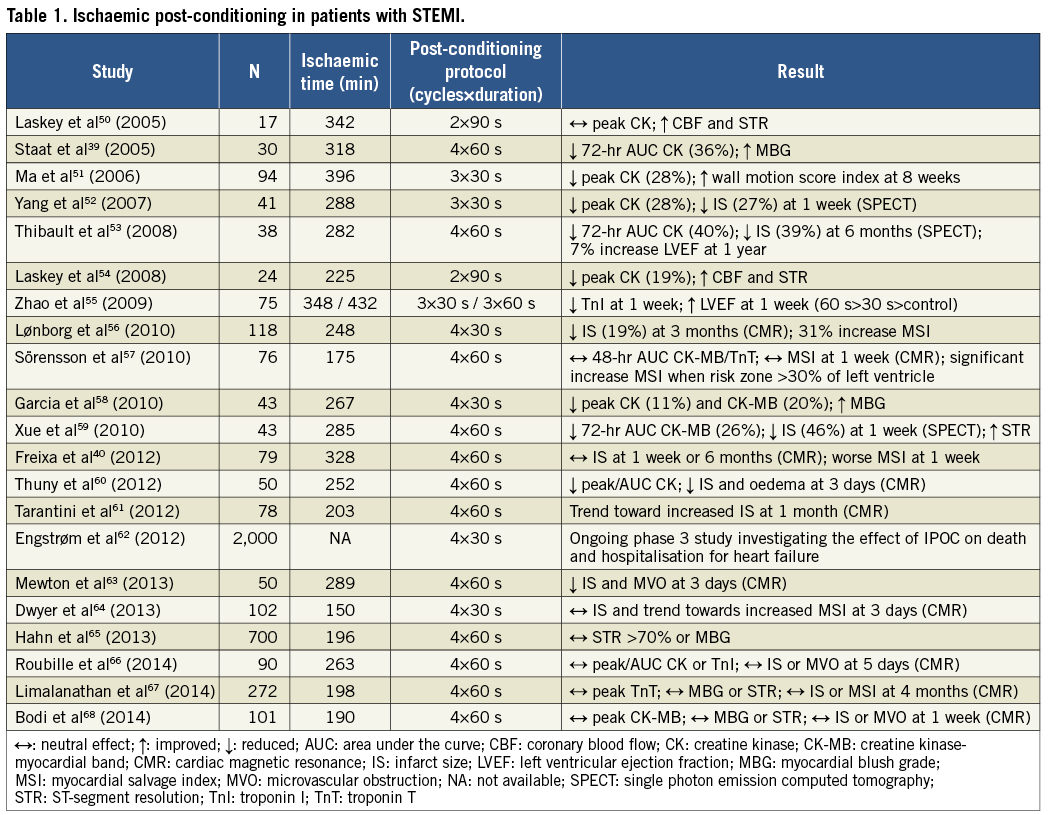
ISCHAEMIC POST-CONDITIONING
A further form of myocardial conditioning is ischaemic post-conditioning38 (Figure 4). In 2003, Zhao et al reported that brief interruptions of coronary blood flow at the onset of reperfusion were also able to induce cardioprotection in canine hearts. The investigators demonstrated that this technique resulted in a 43% reduction in myocardial infarct size following 60 min of left anterior descending artery occlusion38. This concept provided a new strategy of intervention that could be applied at the time of myocardial reperfusion to patients presenting with STEMI, and immediately sparked the interest of clinical investigators.
The first clinical application in patients with STEMI demonstrated that interrupting reperfusion (with four cycles of one-minute low-pressure inflations and deflations of the angioplasty balloon immediately after direct stenting of the infarct-related artery) resulted in a 36% reduction in infarct size as assessed by creatine kinase release over 72 hrs39. However, recent trials have provided inconsistent results40. The value of ischaemic post-conditioning is currently being investigated in, among others, the DANAMI-3 trial (ClinicalTrials.gov identifier NCT01435408). An overview of these clinical studies can be seen in Table 1.
PHARMACOLOGICAL CONDITIONING
Over the last few decades, research into the mechanisms of myocardial conditioning has revealed the function of multiple receptors, signal transduction pathways, and end effectors, all of which are amenable to pharmacological manipulation. These efforts enable drugs to be used to mimic the cardioprotective effects of the different forms of myocardial conditioning. Experimental observations showing that drugs are capable of limiting infarct size when administered just prior to or at the onset of reperfusion have resulted in several clinical studies evaluating these drugs. However, many investigations have yielded negative results, largely due to a lack of consistent preclinical data, poor study design, or delayed drug administration, as previously suggested41.
The signal transduction pathways involved in both pre- and post-conditioning seem to converge at the mitochondria, and studies with agents that are known to preserve mitochondrial function are currently ongoing (CIRCUS and CYCLE, both involving ciclosporin; ClinicalTrials.gov identifiers NCT01502774 and NCT01650662, respectively; EMBRACE, involving Bendavia; ClinicalTrials.gov identifier NCT01572909; and MitoCare, involving TR040303; European Commission FP7 project number 261034). The results of these studies are eagerly awaited to validate the smaller pilot trials conducted so far. In brief, there might be a role for ischaemic conditioning as a strategy with the potential for preserving cell viability after a prolonged ischaemic insult. Although scientifically appealing, the available evidence relating pharmacologic interventions to preconditioning is still insufficient to envisage its imminent clinical applicability. On the other hand, the invasive nature of mechanical post-conditioning interventions limits their use to patients undergoing invasive reperfusion, but the potential of mechanical remote ischaemic preconditioning as obtained with the simple compression of the arm using a pressure cuffing may have relevant clinical implications.
Cell therapy
If enlarging treatment availability to the largest possible number of STEMI patients should be the starting point of efforts to improve reperfusion treatment and survival worldwide, the regeneration of myocardial tissue after cellular death represents the ultimate endpoint, an option that may dramatically transform the natural history of ischaemic heart disease.
Two different forms of cell treatment have been envisaged and developed in this setting. One is the implantation of bone marrow stem cells during or early after primary PCI, and the other is that of implanting the cells during the chronic phase of the disease in patients in whom advanced forms of chronic heart failure after myocardial infarction have evolved.
The demonstration of continued cell division within the adult heart following myocardial infarction42 and the capability of bone marrow-derived progenitor/stem cells (BMSCs) to transdifferentiate into cardiomyocytes, improving left ventricle (LV) function after STEMI, were shown in the mouse model at the end of the 1990s43. Such an amazing finding fuelled continuous research, followed by a substantial number of human clinical trials in the last decade.
Initial studies yielded promising results, in particular data from the first human trial by Strauer et al44. Subsequently, only a modest or no increase in LV function and a reduction in infarct size were observed45-47.
Today, none of the published trials has been able to replicate the magnitude of the functional improvement observed in the animal experiments. Autologous BMSCs, which consist of haematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs), account for the most studied adult stem cells. These are obtained through a bone marrow aspiration under local anaesthesia, isolated and cultivated, using variable protocols in the different trials. Upcoming trials are testing the efficacy of allogeneic MSCs, thus not requiring cell isolation and re-implantation (AMICI trial, number NCT01781390; Prochymal-2 trial, number NCT00877903), as per some promising results derived from animal experiments. Another possible source accounting for the variability of the clinical results derives from the different techniques for percutaneous delivering of the BMSCs into the infarcted area of the left ventricle, with consequent variable retention rates. Cell delivery has been performed by simple anterograde intracoronary injection, by periadventitial administration, and by retrograde transvenous and endoventricular catheters. Such technical differences may in large part account for the diverging findings in these studies. Moreover, other procedural factors have been brought into question such as the timing of cell delivery (ranging from one to 18 days after the acute event), the kinetics of the delivery (from continuous infusion to multiple injections), cell processing protocols and the dose of cells administered.
Moreover, other cell types such as autologous or allogeneic mesenchymal stem cells, adipose tissue-derived regenerative cells, or cardiosphere-derived cells have also been tested in the STEMI setting with some benefits, but these results are strongly limited by the small number of patients treated. In addition, systemic administration of a haematopoietic cytokine, the granulocyte colony-stimulating factor (G-CSF), has been tested in this setting, for its effect of enhanced translocation of BMSCs to the infarcted region post-STEMI. Despite the solid molecular demonstration of the activity of this cytokine, trials involving its use in patients with acute myocardial infarction have shown discrepancy in results, with poor or no improvement in LV function.
Cell delivery immediately after reopening the occluded artery actually carries the interesting perspective of using stem cells derived from young and healthy donors which can be stored on a shelf, readily available directly after primary PCI, and administered in an allogeneic setting, with the obvious logistic simplification. To date, the intracoronary infusion of bone marrow-derived mononuclear cells, although safe, did not enhance cardiac function on MRI-derived parameters, nor did it improve clinical outcome, as recently demonstrated in a meta-analysis of 22 randomised studies48. Conversely, the elective implantation of autologous adult bone marrow-derived stem cells as a treatment for chronic ischaemic heart disease and heart failure provided beneficial clinical effect in terms of mortality and long-term performance status in patients suffering from chronic ischaemic heart disease and heart failure. Cell therapy in this setting has been investigated with similar techniques and methodology as were used in STEMI patients. Intracoronary delivery of BMSCs was tested in the STAR-heart study, which confirmed the beneficial effects of cell therapy in 191 patients with chronic heart failure in terms of improved LV function, improved patients’ exercise capacity and decreased long-term mortality when compared with a control group of 200 patients who declined to undergo active intervention at five-year follow-up49. Similarly, the transendocardial injection of BMSCs has also been shown to be safe with potential beneficial effects, as well as subcutaneous G-CSF administration.
Results of cell therapy in patients with chronic heart failure after STEMI are currently of great interest due to the relevant therapeutic perspectives. The intensive investigation currently ongoing in this field will probably add to our understanding of the real future role of stem cells in the therapeutic horizon of STEMI patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

