Abstract
The field of stent based tissue engineering continues to revolutionise modern medicine by designing novel materials to restore vascular tissue function. Accordingly, the following discussion examines a novel, absorbable, polymeric scaffold engineered in combination with dual therapeutic coating, enabling locally administered temporary scaffolding in the coronary arteries for long term vascular patency and repair. This coronary stent platform consists of an absorbable polymeric material stent structure that incorporates a dual partitioned coating, by means of pro-healing EPC (endothelial progenitor cell) capture technology allowing for rapid endothelial coverage, and an absorbable polymer matrix with sustained elution of sirolimus, a drug controlling neointimal proliferation. This paper provides a brief overview of the various innovations developed by OrbusNeich to create this fully absorbable coronary device platform.
Introduction
For the past two decades, the use of metallic stents has shown that intracoronary stenting is effective in preventing abrupt vessel closure and reducing restenosis after percutaneous coronary intervention (PCI)1-3. A recurring problem with the use of bare metal platforms has been the occurrence of in-stent restenosis over time5-7. In response to this, drug eluting stents (DES) have been developed that provide for the extended release of anti-proliferative and/or anti-inflammatory drugs such as sirolimus and paclitaxel4, both of which result in the reduction of restenosis rates1. Metallic stents coated with antibody cell capture surfaces have also successfully reduced restenosis rates8. Still, the long-term safety of DES remains questionable, mainly due to the possible occurrence of late thrombosis2,3. Fully absorbable polymeric stents represent a promising alternative to metallic stent platforms9,10. In theory, these stents can be used as “temporary” or short term scaffolds, which absorb into the vascular wall after healing is complete. Furthermore, these platforms can be successfully combined with drug eluting coatings and pro-healing EPC capture technology, thereby avoiding the potential complications associated with more long term dual antiplatelet therapy and metallic stents. (Figure 1)
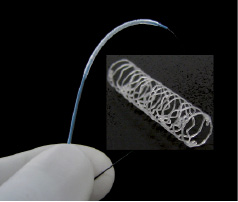
Figure 1. 3.5mm X 18mm hybrid polymer stent; mounted on a 5 Fr compatible PTCA delivery catheter and in a deployed state.
To date, little has been reported on the mechanical aspects of polymer based stents (collapse pressure, stent to vessel ratio, foreshortening, longitudinal flexibility, vascular wall motion compatibility) and the designs of fully absorbable stents11-13. Currently, new polymeric stent material platforms and corresponding stent designs are being developed and evaluated. The initial focus has been on blending polymeric material platforms which are then combined and tailored to enhance stent design performance utilising the unique mechanical properties of polymer materials. A critical hurdle in the development of this platform is evaluating the mechanical functionality of this system when exposed and monitored in both in vitro and in situ environmental conditions. The effects on material mechanical properties, such as the structural integrity of the platform over time, and the biological tolerance of the diminishing material and structural system were evaluated.
To date, prototyped helical stents were made from blended polyester materials of different molecular weights. This particular approach provides a marriage between the polymer material backbone formulations and the mechanical and structural aspects of the stent’s design. In this regard, the short term scaffolding utilising “temporary” stents combined with the two tier coating therapeutic affect, anti proliferative drug elution, and EPC capture, offer great interest as the next evolution in coronary stenting. Therefore, this paper provides a brief overview of the key innovations being developed by OrbusNeich in this area, particularly in regards to polymer material development, mechanical design and testing, and the use and application of partitioned coating technologies.
Polymer material selection
The process of establishing critical performance criteria and material selection is rooted in understanding the entire “life cycle” expectations for the product14. A fully absorbable polymeric stent goes through a “life cycle” that includes synthesis, manufacturing, sterilisation, storage, delivery, healing, breakdown, and absorption. Therefore, it is crucial to consider the performance, design, and manufacturing criteria below in order to obtain a device that effectively balances biocompatibility, degradation kinetics, and mechanical properties15. Design considerations and constraints:
– Degradability that does not provoke tissue overload and other inflammatory responses (breakdown products should possess well understood pharmacokinetics).
– Ability to be fabricated using cast, moulding, extrusion, dip- or spray-coating, using laser cutting or photo etching cutting technologies.
– Ability to further attach or incorporate active ligands and/or drugs onto or within the polymeric device.
– Ability to crimp and maintain the stent onto a balloon delivery system, to deliver the stent to the lesion site, and to expand the device without dislodgement or a stress cracking failure.
– Ability to improve mechanical properties by inducing crystallisation of the materials upon stent deployment (by balloon inflation) under physiologic conditions.
– Ability to retain clinically sufficient radial strength, which aids in overcoming polymer “creep” or yield.
– Ability to withstand sterilisation processes without losing the material’s initial properties.
– Haemodynamic compatibility with blood components and biological fate within the vascular wall.
Taking these and more criteria into consideration, OrbusNeich began focusing its research efforts on the development of stents made from three biocompatible PLA-based polymers (or co-polymers), which were subsequently blended into a semi-crystalline hybrid alloy balancing the performance properties outlined above. Manipulation of the hybrid polymer formulation produces devices with a target elongation that allows for the stent’s deployment, while precluding stress cracking. This polymeric hybrid alloy was also selected for its ability to achieve clinically sufficient strength and resist creep deformation under vascular load. Additionally, when the stent is deployed, the alloy enables sufficient molecular mobility, which facilitates further crystallisation in the stent’s critical deformation zones, thus improving the stent’s overall strength. Controlling the amount and type of crystallinity that results from the material’s composition, structure, and processing, in turn, allows a greater amount of control over the implanted stent’s mechanical properties.
The hybrid alloy’s breakdown mechanism and kinetics may also help avoid localised inflammatory responses caused by tissue overload or mechanical irritation. For instance, it has been demonstrated that slow breakdown kinetics produce a quiescent healing response. In one study, it was demonstrated that a polymer blend of poly(lactide) and a trimethylene carbonate-based copolymer did not cause an inflammatory response in the vessel wall”16,20. The breakdown products of such PLA-based (co)polymer blends are well understood17-19. Additionally, it is generally accepted that these materials degrade primarily via hydrolysis of the ester linkage occurring preferentially in the amorphous regions of semi-crystalline polymers. Again, the control of the amount of crystalline domains in the hybrid alloy allows for a greater control over the degradation rates of the implanted devices.
The development of new PLA-based hybrid platforms reflects the growing need to balance the complex performance demands discussed above. To date, prototype stents drawing upon either homopolymers or single copolymers alone do not appear to have been able to establish a desired multiple “property/performance” balance. In particular, the design demand for thinner strut profiles has prompted the development of designs offering higher strength; and stent crimping and dilation have imposed a need for failure-avoiding toughness. Finally, functional bioabsorbable coatings containing active pharmaceutical ingredients (API’s) and immobilised antibodies have imposed additional material and design requirements.
Polymeric stent design and function
In order to develop and evaluate this new polymeric stent platform, it was critical to establish minimum performance criteria for structural polymer endurance, mechanical stent performance, and biological tissue response, all of which define the overall clinical utility and portfolio of the platform. This design must take into consideration the variables provided in the design window.
Biodegradable stent prototypes were produced from polyester based polymers with different molecular weights. The stent design is based on the OrbusNeich commercially available 316L SST and L605 CoCr platforms of the R stent. The design of this coronary R stent is unique. Its patented dual helix configuration provides omnidirectional flexibility and extremely high radial strength. In addition, the deployed stent geometry enables the individual cells, regardless of where they are located about the stent, to be further dilated for side branch access up to 4.5 mm in diameter.
With the incorporation of these new hybrid polymeric materials as the backbone of the stent platform, it became possible to integrate new functional design elements into the basic R stent design. The limitations of polymer materials present challenges related to maximising deployed radial strength and maintaining mechanical stability over time (in vivo). Furthermore, unlike metal stents, the crimped polymer stents tend to relax the mechanical crimped interface over time, posing challenges related to stent retention24. Lastly, visibility under fluoroscopic observation, OCT or IVUS are virtually nonexistent with these materials.
Radial strength
Polymer formulation, polymeric molecular weight (MW), strut and wall dimensions, coating incorporation, and stent design geometry all have significant effects on the radial collapse pressure and were thus evaluated. The very nature of these novel materials enables dynamic design prototyping, allowing the incorporation of “design elements” to further induce crystalline alignment within the polymer substrate. For example, the creation and stretching effect from the “crimped” to “deployed state” alters the crystallinity within specific elements of the stent design. These embedded design elements within the stent structure create a series of ringlet structures nested within the helical R stent geometry (Figure 2).
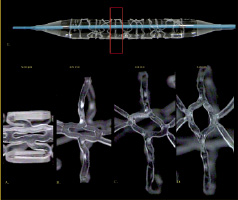
Figure 2. Ringlet design element. Crimped to 3.0 mm-4.0 mm expanded.
Modifications to the stent geometry incorporating specific ringlet structures substantially increased the overall structural integrity and the radial strength of the stents (Figure 3). The force required to compress these rings, perpendicularly oriented along the centre line of the stent axis, is much higher when compared to an expanded “crown like” design element.
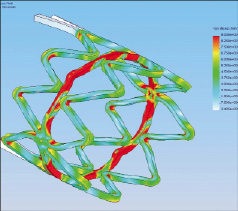
Figure 3. FEA model displayed enhanced crystallisation “red area” within ringlet structure (post-expansion).
The stent produced from combination or material blends has a higher collapse pressure when the load bearing surface area is increased. Furthermore, the design of the stent was also found to a have a significant effect on stent delivery performance and perceived clinical utility.
Stent retention
Polymer materials also enable the use of specific design elements, such as “snap fit” locking mechanisms, to be incorporated directly into the structure of the stent. The elements would not be practical or functional in metallic based platforms. Several iterative snap-fit configurations were evaluated, enabling the stent to be crimped and locked down onto the delivery PTCA balloon catheter. This locking feature serves two purposes; A) enhancing stent-to-balloon retention force, enabling secure delivery of the platform to the lesion site and B) overcoming “polymer crimp relaxation” or “polymer creep” over an extended period of shelf lifetime24. The current platform incorporates three individual micro snap-fit locks equally and circumferentially placed on each end of the stent (Figure 4). During the initial expansion of the stent structure by the PTCA balloon catheter, the snap-fit assemblies are released, allowing for uniform deployment of the stent at the target site.
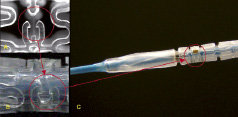
Figure 4. Snap fit locking mechanism A.) Pre-crimped, B.,C.) Engaged post-crimp.
Fluoroscopic and CT visibility
As previously mentioned, the nature of the material precludes visibility under normal fluoroscopic angiography. To enable visualisation under fluoro placement, radiopaque markers are affixed directly onto the polar ends of the stent and incorporated into the locking mechanism. Gold, platinum, and tantalum are all material candidates for such radiopaque markers. The placement of the markers is depicted in Figures 6A and 6B. As the stent degrades over time, the markers remain incorporated within the healed vascular tissue, delineating the polar ends of the stented lesion site. Visualisation of the deployed stent can be characterised by using both spiral CT scan and OCT modalities as depicted in Figures 5 and 6C.
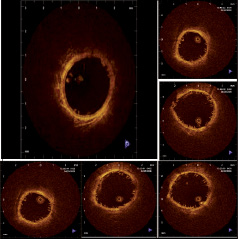
Figure 5. Sectional series of OCT review post deployment in porcine RCA.
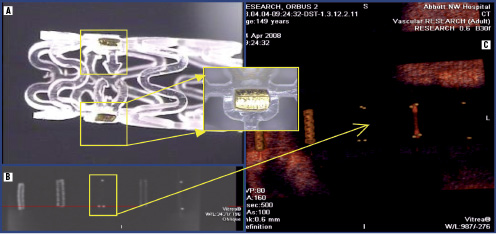
Figure 6. Radiopacity under fluoro and CT visualisation: A. Radiopaque markers positioned within the locking mechanism, B. Fluoroscopic review of markers w/o phantom control, C. Spiral CT visualisation highlighting markers and comparison to control units (polymer, 316L and L605 metallic stents).
Partitioned coating technologies
The absorbable stent platform will also incorporate a partitioned coating technology. OrbusNeich has developed this proprietary coating platform which combines the pro-healing EPC capture technology, found on the commercially available Genous™ Bio-engineered R stent™ for rapidly achieving endothelial coverage and improved functionality28, along with abluminal low dose sirolimus drug elution for control of neointimal proliferation. This metallic stent platform is composed of the OrbusNeich R stent with an abluminal coating of a bioabsorbable polymer matrix formulated with sirolimus for sustained release, and an anti-CD34 antibody cell capture coating on the luminal surface. In pre-clinical studies, the metallic Combo Stent demonstrated significantly lower neointimal hyperplasia, while also showing improved endothelial coverage relative to other commercially available DES. The drug/polymer metallic Combo Stent coating consists of sirolimus loaded in the fully absorbable polymer matrix (Figure 7).
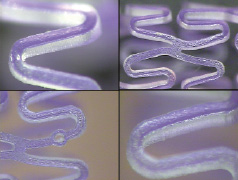
Figure 7. Magnified views of partitioned abluminal coating depicting polymer coating matrix with Crystal Violet Dye prior to immobilised CD 34 Ab coating process.
To promote healing, the anti-CD34 surface modification is applied to the entire Combo Stent such that the luminal surface of the deployed stent presents an immuno-affinity surface to promote the capture of endothelial progenitor cells from circulating blood. By antibody recruitment of the patient’s own EPCs to the site of vascular injury (e.g. the site of a coronary stent implant), an acceleration of the normal endothelialisation process occurs. Rapid establishment of a functioning endothelial layer helps to promote the transformation of the injured site to a healthy state. For example, in the case of coronary stent implantation, rapid re-endothelialisation may reduce inflammation, protect against thrombosis, and even modulate restenosis.
To characterise the antibody activity of anti-CD34 stent surfaces, a cell-based bioassay was developed to verify activity of the bound antibody. This stent bioassay is performed with CD34+ KG-1a cells, a variant of the immortalised human acute myelogenous leukaemia cell line KG-1a, which expresses the CD34 surface antigen. In the bioassay, stents are incubated with KG-1a cells (CCL-246.1, ATCC, Rockville, MD, USA) in phosphate buffered saline (PBS) with 3% bovine serum albumin for one hour with continuous agitation, fixed, and stained with a fluorescent nuclear stain, 4´, 6-diamidino-2-phemlyindoline (DAPI; Sigma, St. Louis, MO, USA). (Figure 9)
The metallic Combo Stent has been shown to be biocompatible and safe through numerous laboratory and pre-clinical implant studies25-27. The degradable polymer-based drug elution technology, the immobilised anti-CD34 antibody cell capture technology, and the combination thereof have been found to be non-toxic, non-irritating, non-immunogenic, and blood-compatible.
In porcine implant studies, Combo Stents (which have half of the total drug content of Cypher) show sirolimus release rates similar to Cypher stents (Figure 8). An equivalent amount of drug is locally delivered to blood vessels compared to Cypher; however, less drug is released into the blood, and distributed to downstream organs compared to Cypher.
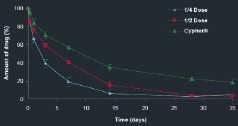
Figure 8. In vitro elution of Combo and Cypher stents (percent of total drug eluted over time). The total drug content is approximately half the dose of the commercially available Cypher stent, but with the same release profile. The figure above shows the in vivo elution profile of the final sterile Combo Stent product as compared to Cypher. The degradation of the polymer occurs around 90 days.
Figure 9. Fluorescence microscopy of KG-1a bound anti-CD34 stents (insert, KG-1a cells with nuclei stained with DAPI, and surface stained with PE-anti-CD34).
Additional porcine implant studies were conducted to evaluate the effects of the metallic Combo Stent test device (abluminal coating) compared to uniformly coated control devices on endothelial coverage and expression of platelet/endothelial cell adhesion molecule (CD31) up to 14 days, and vascular response at 14 and 28 days post-implant in a porcine coronary model utilising imaging, light microscopy, and confocal microscopy techniques. These studies have been submitted for publication by Granada et al and Nakazawa et al.5-27
The partitioned coating technology used to develop the metallic Combo Stent has been shown to be well tolerated and effective in preclinical implant studies, demonstrating sustained drug delivery to the local implant site tissue through pharmacokinetic studies, and controlled neointimal proliferation, while enhancing re-endothelialisation of the implanted stents. This collective body of preclinical research was used to begin a randomised, controlled human clinical trial in Q4 2009. This same technology is transferable to the polymeric material backbone and may be highly effective when applied to the fully absorbable stent platform.
Programme summary
Absorbable stent technologies under development have many specific challenges ahead of them, but to date, all of these platforms known in the field of absorbable stents still lack defined preclinical and clinical evidence demonstrating a clear advantage of this approach over metallic stent systems. Polymeric stents face specific challenges in achieving adequate strength and resistance to structural remodelling over time. OrbusNeich has focused the development of these polymeric based materials and designs into an integrated stent platform and further combined these structures with external partitioned coatings, in order to continue to meet the clinical challenges set forth in today’s cardiovascular practice.
The question remains – will these new polymeric platforms demonstrate the same clinical utility as their metal predecessors, or fall short? This is compounded by the need to reduce local cellular adverse effects of degradation components as these systems are absorbed.
The polymorphic lactide multi-polymer hybrid platform reviewed in this article has the ability to address and balance these complex demands. To date, these prototype stents have demonstrated the ability to blend material, form, and function to establish the desired “property to performance” balance. In particular, with the current metallic based systems setting the performance standard for clinical utility, design limitations such as criteria for thinner strut widths and profiles, require development of higher strength based materials. All of these materials, designs, and manufacturing efforts must also address the stress and deformation applied of the configuration during stent crimping and expansion, all of which pose an additional level of structural integrity to avert strut failure.
In summary, OrbusNeich’s absorbable stent program uniquely combines three technology platforms – a hybrid material, a novel polymer stent design, and proprietary partitioned coatings. The field of biodegradable stents may in theory offer the ideal solution, but with many challenges ahead, the focus will be to broaden the understanding of preclinical safety and performance evidence, as well as continue to critically evaluate the long term clinical safety and efficacy of these novel materials and coatings under the scrutiny of clinical research in the coming years.

