Abstract
Lactide based polymers present a promising class of materials for successful development of fully resorbable stents, thus helping to bring the concept of vascular restoration therapies to life. Not only can these polymers be perfectly tuned to fulfil technical requirements for a fully resorbable stent, they have been proven to be safe materials with a long track record of in vivo biocompatibility in a broad range of medical and pharmaceutical fields. They have a strong regulatory history as well. The polymers degrade through hydrolysis, and are eliminated by the human body through natural pathways via the Krebs cycle.
The polymers can perform a temporary mechanical function, allowing the tissue to heal and resume its original function before the implant looses its mechanical integrity. The mechanical performance of the stent can be achieved through stent design and manufacturing methods, as well as tailoring the properties of the polymer itself.
The resorption time of cardiovascular stents based on these polymers can be tuned –from a polymer perspective– by tailoring the molecular weight, the crystallinity and the hydrophilicity of the polymer. Drug eluting coatings for resorbable stents can be developed from the same family of polymers, tailoring the composition to the desired controlled release of the applicable drug.
To successfully develop resorbable cardiovascular devices an interdisciplinary approach is needed, bringing together polymer chemists and engineers and connecting them with medical device and clinical experts.
Introduction
The application of devices based on resorbable polymers is of ever increasing importance in the medical and pharmaceutical field. As understanding, awareness and familiarity with these types of devices grows, the desire to apply new, innovative products based on this unique family of materials is on the rise. It is no surprise that the use of resorbable polymers is also being considered in the development of cardiovascular stents. Not only do these polymers present a suitable alternative as drug eluting stent coating materials, they are also considered as the core material for fully resorbable stents. Some recent developments in fully resorbable stents show promising results in clinical trials.10,18,19 These results lead to a growing interest and activity into the development of fully resorbable cardiovascular devices. The term Vascular Restoration Therapies (VRT) has been introduced, referring to the restoration of the initial function of the vascular system using these new devices.
This document is intended to provide an introduction to lactide based polymers. First, a short historic overview of polylactide based implants is presented. Then, some characteristics of lactide and glycolide based polymers are given as well as the most important parameters that need to be considered, from a polymer viewpoint, in the design of resorbable implants. Finally, some considerations are presented regarding successful development of resorbable devices based on this polymer family.
History
Commercially available products based on lactide and glycolide derived polymers have been on the market for over 35 years. As a result, they have a strong regulatory history in numerous applications ranging from wound closure, orthopaedic treatments and controlled drug delivery systems to vascular closure devices. In 2006, Jamiolkowsky and Dormier1 estimated the global market for medical devices from lactide and glycolide based polymers to exceed one billion dollars, foreseeing new applications to further increase the commercial importance of these materials.
Wound closure
The first products on the market based on these polymers were surgical sutures for application in wound closure. In the late 1960’s Davis & Geck, (American Cyanamid, MA, USA) developed a polyglycolide based suture marketed under the brand name Dexon.2 In 1974, Ethicon (Ethicon, Inc., a Johnson & Johnson Company, Somerville, NJ, USA) came to the market with their Vicryl Suture based on a copolymer of lactide and glycolide. To date, these types of sutures still dominate the market for wound closure. Currently, resorbable sutures are available with tailored properties regarding strength, strength retention and resorption time. Dependent on the required functionality of the suture, complete resorption times can be varied from less than two weeks up to two years.1,3
Furthermore, there are several products combining the unique features of resorbable devices with incorporated drugs, i.e. drug-device combinations. In 2003, Ethicon launched a drug-coated suture under the brand name Vicryl Plus. This suture is coated with anti-bacterial layer of Triclosan which is released during the first days after implantation to offer protection against bacterial colonisation on the suture.
Orthopaedics
In 1977, Törmälä developed the first glycolide and lactide based orthopaedic implant devices. The first-in-man with this class of orthopaedic implants was performed in 1984 by Rokkanen.4 These implants were developed as fracture fixation devices. To date, numerous products are developed and commercialised for a broad range of orthopaedic applications ranging from sports medicine and trauma to spinal surgery. An example of an orthopaedic device with a combined drug delivery function was developed at the Tampere University of Technology in Finland. Ciprofloxacin-releasing fixation screws and pins developed from this research have had positive clinical results and are further developed by Bioretec (Tampere, Finland).5
Drug delivery
Lactide and glycolide based polymers are also used in the formulation of controlled release drug delivery systems. Based on the ability of these polymers to incorporate drugs that will ultimately be released after injection or implantation, a variety of parenteral controlled release systems were developed and commercialised. In 1986, the first product launched in clinics in Europe was Decapeptyl LP, developed by Southern Research Institute (Birmingham, AL, USA).6
Other well-known examples are AstraZenaca’s (London, UK) implant Zoladex which has been developed in the early 1980’s, Novartis (Basel, Switzerland) injectable Sandostatine LAR and Risperdal Consta by Janssen Pharmaceutical (Beerse, Belgium, a division of Johnson & Johnson).
Cardiovascular
In 1996 the Angio-Seal vascular closure device based on a copolymer of lactide and glycolide was introduced to the market. This product was developed by Kensey Nash and marketed by St.Jude Medical (St. Jude Medical, St. Paul, MN, USA), and up to this date millions of these devices are utilised after cardiac catheterisation or angioplasty. Over 300 studies have proven that Angio-Seal is safe and effective in a broad range of patients and procedures.7-9
Cardiovascular stents present a new application area for lactide based polymers where the unique ability of these resorbable materials to serve both as a drug eluting coating as well as a mechanical temporary core material scaffold are combined.10
Lactide based polymers
The products that were described in the previous section are manufactured from lactide based polymers. These polymers have a long track record of being safe and highly biocompatible and can be tailored to the application due to their flexibility in property design. Further advantages are numerous. The materials will disappear while the original function of the body is restored. This natural, patient compliant, solution eliminates the need for secondary surgery to remove the device. Implant devices prevent stress shielding in soft tissues and are load-sharing with bone, which facilitates the healing process of the body. In addition, another advantage over metals is that the polymers are radiolucent, therefore preventing image interference.
PURAC biomaterials has developed, manufactured and marketed lactide based polymers under the PURASORB brand name for over 35 years, facilitating the successful development of resorbable stents with its polymer expertise through interaction with select players and key opinion leaders. In the next sections the concept of temporary functionality is discussed along with the possibility of influencing the resorption rate of the device.
Temporary functionality
The main feature of polylactide based implants, as compared to metallic implants, is that they can fulfil a temporary function in the human body. After the initial function of the body’s defect is restored, the implant can degrade, leaving no foreign body material in vivo and allowing the body to regain its initial, healthy, condition. These implants fulfil the functionality requirements that must be met: either mechanical for devices, or diffusion characteristics for controlled release drug delivery implants.
In Figure 1 the required temporary functionality is schematically depicted for a mechanical property requirement. In vivo, the implant (~polymer) has to temporarily take over the functionality of the corresponding tissue. Overtime, during the so-called healing phase, the tissue restores its initial functionality, while the polymers’ functionality gradually decreases.
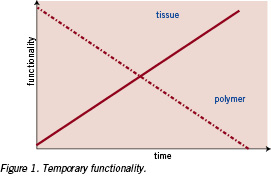
In practice, the strength of the implant has to be high enough to function properly over the complete healing time. Therefore, the mechanical integrity of the temporary implant combined with the increasing strength of the healing tissue has to allow the tissue to serve its function as normal. In most applications, the primary requirement of the implant is to serve a temporary mechanical functionality. Polylactide based implants, degrade over time as represented in Figure 2.11
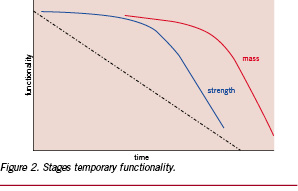
After implantation, chemical cleavage of the polymer backbone results in a gradual decrease of the polymer chain length. Below a specific chain length, the mechanical integrity of the implant decreases relatively fast. The mass of the implant decreases only after a further reduction in polymer chain length. The higher the required mechanical integrity (~strength retention) the longer it takes for complete mass loss occurs, as these two parameters are correlated.1,12
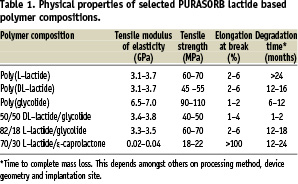
The mechanical properties of resorbable lactide based polymers can be tuned using various monomeric building blocks such as L-lactide, D-lactide, DL-lactide, glycolide and ε-caprolactone. Table 1 shows that the initial mechanical properties vary when different monomeric building blocks are used. As an example, the flexibility, from a polymer perspective, can be tuned from the flexible 70/30 L-lactide/ε-caprolactone-copolymer towards the more rigid poly(L-lactide). Moreover, the mechanical properties can also be influenced via processing techniques and/or design of the device itself.
Degradation of lactide based polymers
Degradation stages
As explained by Perrin and English12 , as well as Kronenthal13, from a chemical standpoint, resorbable implants are thought to undergo five stages of degradation. These stages are not discrete and overlap.
1. HYDRATION
When the implant is placed in the body, hydration of the polymer begins, absorbing water from the surrounding tissue. As polylactides and polglycolides are relatively hydrophilic in nature water penetrates deeply into the interior of the implant. This is typical for a bulk-type of degradation.
2. DEPOLYMERISATION / CHEMICAL CLEAVAGE OF THE POLYMER BACKBONE
Hydrolysis is the basic mode of degradation since water reacts with the covalent bonds and segments the polymer chain into smaller polymer chains, decreasing the molecular weight.
3. LOSS OF MASS INTEGRITY
The loss of mass occurs when the implant essentially has no cohesive strength and the polymer starts to fragment into segments of low molecular weight polymer.
4. ABSORPTION
Absorption via assimilation by phagocytes or further hydrolysis leads to soluble monomeric anions (such as L-lactate) which dissolves into the intercellular fluid.
5. ELIMINATION
The soluble L-lactate is converted into pyruvate, which enters the Krebs-cycle. There it is converted into carbon dioxide and water, finally resulting in complete resorption of the implant.14
Influencing the rate of degradation
From a polymer perspective there are three main factors that influence the rate of degradation and the resorption cycle as described above: the polymer chain length, the hydrophilicity and the crystallinity. These factors are further discussed below.
A. POLYMER CHAIN LENGTH
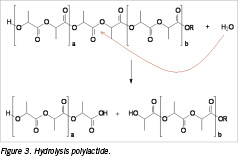
In the first two stages of degradation, water molecules penetrate the implant and hydrolytic chain cleavage occurs. The polymer chain length (~ molecular weight) has a strong influence on the degradation time of the implant as a higher initial chain length results in a longer resorption time of the implant. In Figure 3 the chemical cleavage of the polymeric backbone of polylactide is shown.
An example of the difference in degradation time as a function of the polymer chain length is presented by Chawla et al.15 They show a poly(DL-lactide) having a molecular weight of 90 kg/mol, respectively 300 kg/mol degraded in vivo after 48 weeks with a weight loss percentage of 21%, respectively 7%.
B. HYDROPHILICITY
The hydrophilicity of the implant has a significant influence on the rate of stages one and two as well. The more hydrophilic the polymer, the higher and faster water uptake will occur and the faster the chemical cleavage of the polymer backbone will be.
In Figure 4 the repeating monomeric unit of respectively polyglycolide and polylactide are shown. The methyl side group in polylactide makes the polymer more hydrophobic and sterically less accessible for hydrolytic cleavage of the polymer backbone. As a results, a semi-crystalline polyglycolide degrades faster compared to a semi-crystalline poly(L-lactide).12
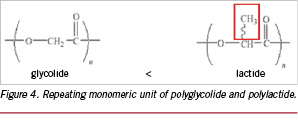
The difference in degradation behaviour between copolymers of lactide and glycolide was presented by Landes et al.16 They showed that a copolymer of 85% L-lactide and 15% of glycolide fully degrades in vivo within one year, whereas a 85% L-lactide 15% D-lactide copolymer degrades within two years.
C. CRYSTALLINITY
The crystallinity of the implant significantly affects the first three stages of degradation. Hydration of amorphous segments of the polymer occurs faster compared to crystalline segments. A schematic representation of an amorphous and a semi-crystalline polymer is shown in Figure 5. The ordered polymer chains in the crystalline segments of the semi-crystalline polymer and the random polymer chains in the amorphous segment are presented.
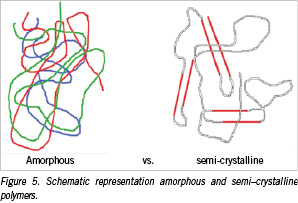
Water penetrates the amorphous regions, which are less densely packed and are therefore more susceptible for hydration. A semi-crystalline polymer always exhibits a certain percentage of amorphous segments. The crystalline regions minimise water uptake. A decrease in hydrolysis also results in less swelling (~ deformation) of the implant and an increased strength retention. As a result, semi-crystalline polymers are preferred to serve a mechanical property, whereas amorphous polymers allow a more uniform dispersion of a drug and are therefore preferred for usage in controlled drug release systems.
Resorption of copolymers is normally faster than the corresponding homopolymers as the overall crystallinity in copolymers is lower providing a more open micro-structure allowing easier water penetration.12,17
Developing resorbable cardiovascular devices
The polymer is an important parameter in the final performance of the product. However, other parameters such as the processing and manufacturing method, the device design, the indication and the patient themselves determine to a great degree the efficiency of the implant.1 The choice of polymer, processing method, design and site of implantation are interconnected in achieving and controlling the performance of a resorbable implant. Developing successful resorbable cardiovascular devices therefore calls for an interdisciplinary approach, bringing together polymer chemists and engineers with device and clinical experts.
In vascular restoration therapies the correct choice of material is of paramount importance. The required properties for a drug-eluting coating are not similar to those for the stent-core. A drug eluting stent normally requires a reliable and uniform drug elution profile. The stent-core has to maintain its mechanical property during the healing phase, with a gradual transition of the structural functionality from the device to the vessel wall. Both the coating and the core material have to function, and ultimately be eliminated from the human body, without causing any adverse reactions.
The preferred type of polymers for a drug eluting coating are amorphous polylactide based polymers with a relatively low molecular weight. They allow for a homogeneous dispersion of the drug and uniform degradation. Furthermore, they allow for short to intermediate release times, from days up to several months. When applied as a thin coating layer these polymers will be fully resorbed after several weeks or months.10,18-20
The recommended polymers for the stent core are semi-crystalline polymers of the polylactide family. These have a relatively high initial strength and high strength retention in which the crystalline regions serve as load-bearing elements. To further optimise the mechanical performance of stents, from a polymer perspective, blends of different polylactides are being investigated as well as the addition of other monomers to the composition to allow for more flexibility and toughness of the resulting device.
Conclusions
Lactide based polymers present a promising class of materials for successful development of fully resorbable stents, and thus can bring the concept of vascular restoration therapies to life. Not only can these polymers be perfectly tuned to fulfil technical requirements for a fully resorbable stent, they have been proven to be safe with a long track record of in vivo biocompatibility in a broad range of medical and pharmaceutical fields as well as having a strong regulatory history. The polymers degrade through hydrolysis, and are eliminated by the human body through natural pathways via the Krebs cycle.
The polymers can perform a temporary mechanical function, allowing the tissue to heal and resume its original function, before the implant loses its mechanical integrity. The mechanical performance of the stent can be achieved through stent design and manufacturing method as well as tailoring the properties of the polymer itself.
The resorption time of cardiovascular stents based on these polymers can be tuned, from a polymer perspective, by tailoring the molecular weight, the crystallinity and the hydrophilicity of the polymer. Drug eluting coatings for resorbable stents can be developed from the same family of polymers, tailoring the composition to the desired controlled release of the applicable drug.
To successfully develop resorbable cardiovascular devices an interdisciplinary approach is needed, bringing together polymer chemists and engineers and connecting them with medical device and clinical experts.

