Abstract
Today the domains of life-respecting, degradable therapeutic materials and devices are among the most attractive areas in polymer science. Increasing attention is being paid to polymeric compounds that can be bioresorbable, i.e., degradable and ideally excreted or bio-assimilated – suitable to treat human diseases or trauma that require therapeutic assistance for a limited period of time, namely the healing period. Basically, biopolymers are of interest because of their inherent biodegradability. However, other characteristics limit their applications in the human body. Artificial polymers, i.e., polymers of non-natural origin, can be degradable in vivo and thus serve in time-limited biomedical or pharmacological therapy. The potential of these degradable polymers is discussed with respect to bioresorbability, with a special attention to polymers of the poly (α-hydroxy acid) type.
Introduction
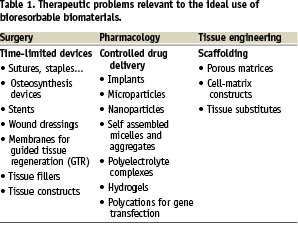
Human beings started using available materials for therapeutic purposes several thousand years ago. Since then, the strategy of the therapist has always been the same: trying to take advantage of any novel materials to treat diseases or trauma. It is the reason why metals, alloys, ceramics – and also biopolymers such as wood, cellulose or proteins – have been used or proposed as therapeutic materials long before the concept of biomaterial was defined. During the last fifty years, attention was increasingly paid to confronting the various properties of materials to specifications of a given application in an attempt to achieve better matching. Among the disease or trauma-related therapeutic problems requiring the use of prosthesis, distinction was made between those requiring a permanent aid to replace an organ or a function definitively lost, and those required for a limited period of time, namely the healing time. Whereas permanent prostheses require biostable materials, time-limited therapeutic aids should be ideally eliminated from the body after healing in order to avoid the storage of foreign compounds. Table 1 lists some of the therapeutic applications that are relevant to time limited therapeutic aids.
Some inorganic compounds can de degraded in the human body. However, polymers are attractive to design degradable therapeutic devices because polymer science offers outstanding possibilities to combine various small building blocks, or repeating units, to generate macromolecular systems that can cover large property ranges. Mother Nature took very much advantage of this particularity to make proteins, polysaccharides and polynucleotides, the three major types of macromolecules the human body is made of. This is also the reason why man-made artificial polymers have invaded most of the human activities, including therapy.
In the field of therapeutic polymers degradable in vivo, the first commercialised devices were sutures made from glycolic and lactic acids that were referred to as “absorbable sutures”1. Actually, the term “absorbable” was not appropriate because absorption does not mean “degradation”, and even less, “elimination from the body”. In general, the phenomenon of absorption reflects the disappearance of a compound into another substance like water into a sponge. In the case of polymers, disappearance is not enough because high molecular mass molecules are trapped between skin and mucosa and can hardly cross physiological barriers that separate the parental compartments. Therefore, a polymeric device that is no longer visible at the site of implantation after some time period is not necessarily degraded and therefore eliminated from the body. It can be only dissolved, macromolecules being thus dispersed and stored within the body. What is necessary, is a cleavage of the main chain of macromolecules, revealed by a decrease of the molecular mass. This cleavage forms small degradation by-products more or less rapidly, and these can then be eliminated through biological pathways. There are only two such pathways to eliminate low molecular mass by-products: the kidneys, provided these residues are soluble in the blood; and the lungs, after metabolism to CO2 and H2O, generally associated to assimilation and biomass formation. The cleavage of macromolecules can result from an abiotic chemical reaction or from cell and tissue activities. To clearly distinguish the various possible situations – as well as allowing specialists from different disciplines the possibility of talking and understanding the same language – “degradation, degradable and degradability” are to be used in the case of unknown or abiotic mechanisms, whereas “biodegradability, biodegradation and biodegradability” should be kept to cell-mediated degradation. Consequently, degradation caused by isolated enzymes under abiotic conditions should not be referred to as “biodegradable” because such commercial enzymes are not necessarily present in vivo at the site of implantation. To avoid the confusion often present in literature, and clearly distinguish between degradation or biodegradation – that reflect degradation mechanisms only – from total elimination by excretion and assimilation, the concept of “bioresorption” was introduced2.
The rest of this article is devoted to correcting some of the beliefs, as well as emphasising some facts, regarding the different levels and mechanisms of degradation in the context of bioresorption.
Bioresorbable polymers in therapy
Today, any disease or trauma that requires the use of a therapeutic system for a limited period of time is relevant to the use of bioresorbable materials. At the very top of the elimination of foreign substances introduced in parenteral compartments, degradation of macromolecules has another advantage. The resulting progressive decrease of initial properties allows better healing, because cells and tissues find again the surrounding stresses the prosthetic device was deviating or masking3. It is well known that the reconstruction (healing) of an injured tissue proceeds through three successive steps: inflammation post-injury, consolidation and remodelling, shown in schematic form in Figure 1.
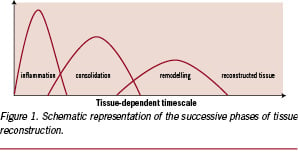
A similar sequence of events is observed in the case of injuries caused by the implantation of a prosthetic device and, of course, of a device made of – or including – degradable polymers. For cells to find again surrounding stresses and reconstruct tissues correctly, a matching decrease of properties and tissue reconstruction appears to be very important, and has to occur during the consolidation and/or remodelling phase; i.e., before remodelling is completed and cell activity is stabilised again. If the degradation is too slow, the device is seen as biostable. If it is too fast, degradation has not much effect on the tissue reconstruction. Figure 2 (left) exemplifies the significance of the degradation rate on the replacement of bioresorbable bone plates made of poly(lactic acid) stereocopolymers having different lifetimes.
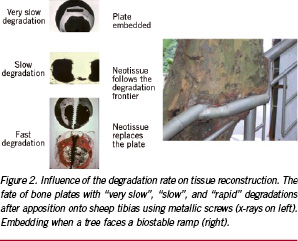
As one can see from the x-rays, the long lasting device (transparent and thus black) is seen as biostable, embedded within new bony tissue, whereas a rapidly degraded analogue is replaced by woven bone. The intermediate compounds show that tissue reconstruction follows the loss of substance. Figure 2 (right) shows this is not a behaviour specific to animal tissue since a tree also embedded the biostable ramp it cannot degrade.
Ideally, a bioresorbable polymeric biomaterial must be biocompatible, biofunctional, degradable at a rate appropriate to allow replacement by a neo-tissue and eliminated from the human body at the end.
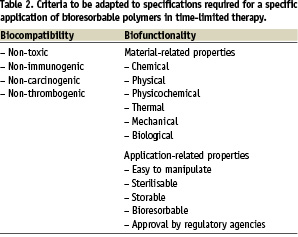
However, one should not forget that bioresorbability is only one of the requirements to be fulfilled. The others are those typical of biomaterials (Table 2). Among these, sterilisation and long term storage can be problematic for devices made of degradable or biodegradable polymers. Most of the approved methods of sterilisation are aggressive against organic macromolecules and degrade many of them (radiations) and/or alter dimensions (heat). If they are absorbed, chemicals like ethylene oxide or formaldehyde can cause property modifications (chemical defects along the chains, lowering of the glass transition temperature below body temperature with the risk of deformations due to creeping or chain relaxation phenomena) or leave toxic residues absorbed within the matrix. Therefore, suitable requirements listed in Table 2 should be systematically taken into account in a strategy supposed to bring a polymeric bioresorbable device up to the market.
Biopolymers
Because macromolecular compounds synthesised by Mother Nature are usually biodegradable – i.e., degraded by cell-mediated biological processes – biopolymers are often regarded as suitable compounds to make bioresorbable therapeutic devices. However, biopolymers are rather difficult to process, exhibit poorly reproducible properties, can be immunogenic and are often difficult to store because of their sensitivity to humidity and to micro-organisms. Table 3 lists the major biopolymers.
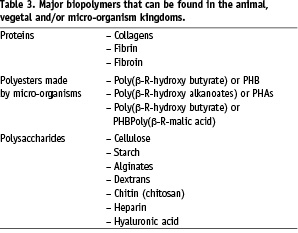
These shortcomings are rather well known. However, there are some other statements transmitted in literature that are wrong or, at the very least, inappropriate. In many instances, biopolymers are chemically modified generally to improve some properties (mechanical, life-time, etc.). There is, for instance, the case for collagen and hyaluronic acid that are usually more or less cross-linked but, nevertheless, presented as collagen and hyaluronic acid.
One should keep in mind that biodegradation disappears rapidly when the degree of chemical modification increases and, also, that some cross-linkers can be highly toxic. It is the case of glutaraldehyde that can be released from cross-linked collagen in the absence of stabilisation of imine linkages by hydrogenation.
On the other hand, some artificial polymers are presented as biodegradable in the absence of demonstration. As a result, the conclusions one can find in literature are sometimes questionable. For instance, chitosan is generally presented as a biopolymer and as biodegradable in vivo. Firstly, chitosan is not a biopolymer. Chitosan is a generic name for a large family of derivatives obtained by deacetylation of the chitin biopolymer. The properties of deacetylated chitin depend very much on the degree of deacetylation. Some members of this copolymer family are soluble in aqueous media, and some are not. Secondly, if some chitosans can be degraded partially in vivo and enzymatically in vitro, their bioresorbability has never been demonstrated4.
Poly(β-R-(hydroxy butanoate) and poly(β-R-hydroxy alkanoate)s are also generally presented as biodegradable polymers in the literature. These biopolymers, made by bacteria, are actually biodegradable by outdoor micro-organisms only. The human body does not have the necessary specific depolymerases to degrade these polymers and thus they are only hydrolytically degradable in the animal kingdom. Their rate of hydrolysis is slow and disappearance of derived devices can hardly match tissue reconstruction machineries, even those of bony tissues.
These few examples show that one should be very careful in the use of biopolymers or chemically modified biopolymers to design bioresorbable therapeutic devices or macromolecules. The available literature is often confusing, contributing to the diffusion of inappropriate statements about compounds that generally lack precise identification and characterisation4.
Artificial (non-natural) polymers
As a result of many of the advances in organic chemistry over the last century, artificial polymers were invented. In the last seventy years, this novel type of materials outclassed biopolymers in most of their industrial and domestic applications. The same evolution was observed in therapy, since surgeons and pharmacologists tried to take advantages of synthetic polymers as soon as they became available. However, one of the main criteria that made domestic and industrial synthetic polymers better than biopolymers is biostability. This property precluded the use of classical polymers to make degradable or biodegradable therapeutic devices. It is the reason why, during these last decades, scientists have been searching for novel artificial polymers that can degrade or biodegrade when allowed to work in contact with living tissues. Many artificial polymers have been found biocompatible and degradable or biodegradable in an animal body.
There are many different polymers presented as biodegradable or absorbable in literature. Examples are given in Table 4.
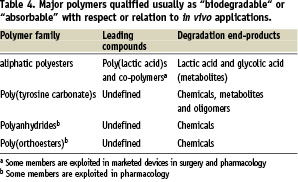
At this point, it is of interest to note that a degradable or biodegradable polymeric device starts as a biomaterial but ends up as small molecules relevant to the field, and thus enters the realm of pharmacological principles and regulations. This has not yet been fully taken into account by regulatory agencies, but the trend now exists. In this regard, artificial polymers that generate metabolites as degradation by-products are preferable for the sake of safe elimination or assimilation5. One can consider macromolecucles made of pro-metabolite repeating units as artificial biopolymers6.
In the general literature, and in clinical applications as well, members of the aliphatic polyesters family are the most frequently mentioned as in vivo-degradable (Table 5).
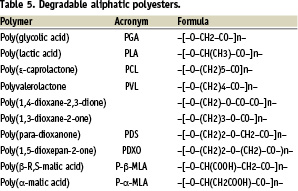
Most aliphatic polyesters are generally introduced as biodegradable7. Actually, most of them are hydrolytically degradable only. Polycaprolactone (PCL) is often mentioned as biodegradable on the basis of enzymatic degradation by lipases in vitro and of biodegradation by micro-organisms in vitro, as well as in the environment. However, although PCL is enzymatically degradable by lipases in vitro, it is not biodegradable in vivo. This is probably because, like in the cases of PHB and PHAs, the active enzymes are not available. Therefore, PCL, like PHB and PHAs, degrades hydrolytically only. Because these three types of polyesters are very hydrophobic and highly crystalline, two properties that act against hydrolysis, they degrade very slowly in vivo and are preferably suitable for very long-term applications. So far PHAs have not received any commercial development as biomaterials. PCL is used for long term, sustained drug delivery.8
Among the other hydrolytically-degradable aliphatic polyesters, not all are of practical interest because they cannot fulfil the requirements of any specific application. For instance, natural and artificial poly(β-malic acid)s are soluble in water, and thus cannot be used to make solid state devices. However, they look very interesting as sources of macromolecular pro-drugs having a bioresorbable backbone. Hydrophobic poly(β-malic acid) benzyl esters can be used to make solid state devices, but they are no longer degradable because of hydrophobicity8.
Finally, the most attractive bioresorbable polymers are poly(α-hydroxy acid)s derived from lactic and glycolic acids, thanks to chirality and to copolymerisation, two structural characteristics largely used by Mother Nature to diversify biopolymers.
Poly(α-hydroxyacid)s derived from lactic and glycolic acids
As mentioned before, the main advantage of this family is an outstanding versatility due to chirality and copolymerisation between glycolic acid and lactic acid stereomers (Table 6). Combined with the influence of molecular mass on polymer properties, these structure-determining tools offer means to obtain bioresorbable compounds going from wax to hard solids, having a life-time from a few weeks to several years, and being amorphous or semi-crystalline. The main properties of poly(glycolic acid) and (poly(lactic acid)s were identified many years ago1,9-11.
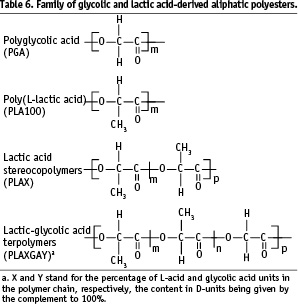
The family can be enlarged by copolymerisation to form copolymers with small segments of water soluble polymer chain like poly(ethylene glycol)s, (PEGs) provided the molecular mass of the released segments permit elimination through kidneys in order to respect the concept of bioresorption.
In this versatile family, PGA and PLA8GA92 (good fibre forming and rapidly degraded) are used in commercial “absorbable” (bioresorbable) sutures and meshes; i.e., for soft tissues. Semi-crystalline PLA98 and PLA100 are used to make osteosynthesis bioresorbable devices like plates, screws, staples, pins, intervertebral cages, etc. PLA100 and PLA stereocopolymers are under investigation to replace metallic stents by bioresorbable ones. Amorphous PLA and PLAGA are preferably used to make drug delivery devices (implants, films, microparticles, nanoparticles) where a drug is temporarily entrapped within a bioresorbable matrix. PLA, PLAGA and PLA-PEG-PLA triblock copolymers are being investigated as bioresorbable porous scaffolds in tissue engineering. PLA-type polymers are now produced in large scale to make compostable packaging. However, these last polymers are formulated with additives and are thus not recommended for biomedical applications.
Insofar as biomedical applications are concerned, the hydrolytic degradation of poly(α-hydroxy acid)s can raise major problems: adjustment, control and reproducibility of properties on the one hand, and possible formation of tiny long lasting crystalline residues that can be dramatically inflammatory, on the other hand. Table 7 lists the major factors that can affect the degradation characteristics of a poly(α-hydroxy acid). Some speed-up degradation, others slow it down12.
Controlling the balance of their accelerating or slowing effects is essential, however, it is rather difficult because of the interdependence between many of these factors. The complexity comes from various phenomena: i) the hydrolytic degradation of poly(α-hydroxy acid)s that is auto-catalysed by carboxylic groups normally present at one chain end; ii) the diffusion of oligomeric degradation by-products within the amorphous phase of the matrix; iii) the solubility of the oligomers when they become small enough to be soluble in the surrounding liquid medium. Most important is the fact that degradation of copolymer main chains is not homogeneous, weaker zones being degraded preferentially with composition changes that leave the less degradable part as long lasting – and sometime inflammatory – crystalline residues13. This phenomenon accounts for the observation of successive inflammatory reactions with tissue-dependent time scales: one right after implantation as normal, one later on when soluble oligomers start being released from the matrix and a last one much later if tiny crystalline residues are formed. It is sometime claimed that released acidic compounds are the source of inflammation, despite the buffering effect of body fluids. A more credible alternative is a tissue reaction triggered by the release of soluble oligomers, given the fact that release stretches over weeks to months, and in some cases, years. Rapidly degrading PLAGAs are much more inflammatory than slowly degrading PLA100 (PLLA).
Another striking feature of poly(α-hydroxy acid)s is the size-dependence of the degradation rate. Unexpectedly, the lifetime of thin or tiny devices is much greater than that of thick ones. This particularity was related to the fact that auto-catalysing soluble oligomers are released before total degradation from tiny matrices, whereas they are completely degraded before getting to the surface of thick matrices14. This is a general phenomenon that can be observed more or less in all hydrolytically degradable polymers, depending on the ratio between diffusion and hydrolysis rates. These phenomena are well documented in literature.
Insofar as actual therapeutic applications are concerned, sutures made of PLA, PLAGA, PDS, etc.; staples, pins and bone fracture fixation devices made of various PLAX stereocopolymers and PLAGA copolymers; interference screws made of PLAX and PLAGA sterocopolymers to fix autologous anterior cruciate ligament prostheses15; membranes made of PLAX stereocopolymers for guided tissue regeneration and orbital floors, are all marketed. The literature mentions many examples of numerous other devices that are still at the research level, degradable and bioresorbable stents are good examples16,17.
Conclusion
The biomaterials field has come a long way from its empirical beginnings. From this rapid survey of polymer degradation and degradable polymers one can conclude that degradation characteristics required by specific applications are rather difficult to fulfil. Therefore, there is no universal bioresorbable polymer. Each therapeutic problem requires a specific solution and a suitable compromise between the structural, chemical, physico-chemical as well as biological factors that can affect the degradation and the in vivo fate of a time-limited polymeric prosthesis. This is one of the reasons – if not the major one – that the number of commercially successful systems in medicine and in pharmacology is still rather small. To have a chance at developing a polymeric bioresorbable device up to commercialisation, it is essential to include in your strategy from the very beginning, biocompatibility, biofunctionality and bioresorbability requirements specific to and for the targeted applications.
Acknowledgements
The author is indebted to all his co-workers who contributed to bring the understanding of this field to its present stage.

